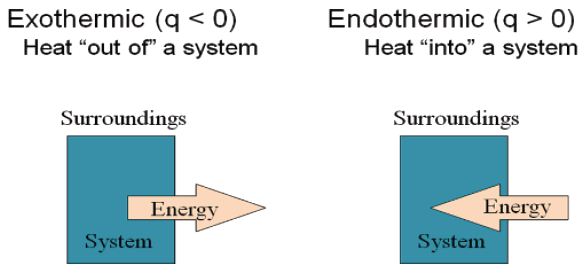
Heat can be defined as the energy that flows into or out of a system because of a difference in temperature between the system and its surroundings. The Heat energy is transferred from a region of higher temperature to one of lower temperature; once the temperatures become equal, heat flow stops.
Temperature: Remember, heat is not the same as temperature. Temperature is directly related to the average kinetic energy of the particles. The temperature change when a certain quantity of heat is transferred will depend on the amount of substance present The Temperature of the system can also change if the volume or pressure are changed.
Heat and Chemical Reactions: In equations Heat is denoted by the symbol q and is positive if heat is absorbed by the system. On the other hand, q is negative if heat is evolved by the system.
Heat and Work
Part of the energy released can be used to move something (“do work”).
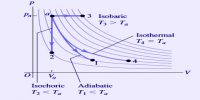
E.g. if a gas is produced during the reaction the volume changes, and work has to be done to push back the atmosphere. This amount of work is PΔV, which subsequently changes the internal energy U.