Immiscible Liquid Pairs: Steam Distillation
There are pairs of liquids which are insoluble in each other. When mixed in any proportion they separate into two layers. Such liquids are said to be immiscible with each other. Examples are: carbon tetrachloride/water, mercury/water, nitrobenzene/water, aniline/water.
If a pair of immiscible liquids is stirred, the vapour pressure above the mixture is found to be equal to the sum of the vapour pressure of the components A and B. Thus
Ptotal = P0A + P0B
The total vapour pressure of the mixture is thus higher than the vapour pressure of any of the two components. A consequence of this is that the mixture boils at a lower temperature than any of the two components. This is shown in Figure 1. Here the vapour pressure of the two components A and water and that of the mixture at different temperatures are plotted. The vapour pressure of all three liquids increase with increasing temperature. When the total vapour pressure equals 1.0 atm the liquid will boil.
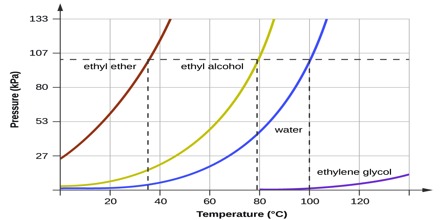
Fig: Vapour pressures of water and octane and their mixture at different temperatures
As can be seen the mixture boils at a lower temperature than any of the pure liquids. This property of such mixtures has two used to separate/purify many organic liquids which are immiscible with water. The method is known as steam distillation.
A diagram of an apparatus used for steam distillation is shown in Figure 2. It consists of a round-bottomed flask F, having a glass inlet tube which reaches almost to the bottom of the flask and is connected to a steam generator G. The flask has also a glass outlet tube connected to a Liebig condenser.
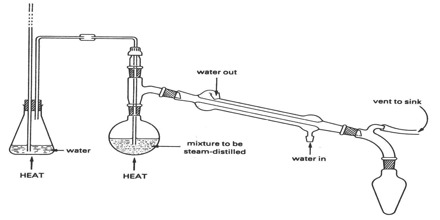
Figure: Apparatus for steam distillation
The material to be steam-distilled is placed in F and a vigorous current of steam blown in from G. The mixture is thus rapidly heated and the vapour of the organic compound mixed with steam passes over and condensed in E. The organic compound can be separated from water with the help of a separating funnel and dried by suitable means.