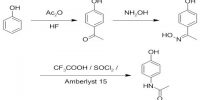
Hydrogen bonding is important in numerous chemical procedures. Hydrogen bonding is dependable for water’s inimitable solvent capabilities.
i) Life would have been impossible without liquid water which is the result of intermolecular H-bonding in it.
ii) Hydrogen bonding increase the rigidity and strength of wood fibres and thus makes it an article of great utility to meet requirements of housing, furniture, etc.
iii) The cotton, silk or synthetic fibres also own their rigidity and tensile strength to hydrogen bonding.
iv) Most of our food materials such as carbohydrates and proteins also consist of hydrogen bonding.
v) Hydrogen bonding also exists in various tissues, organs, skin, blood and bones.