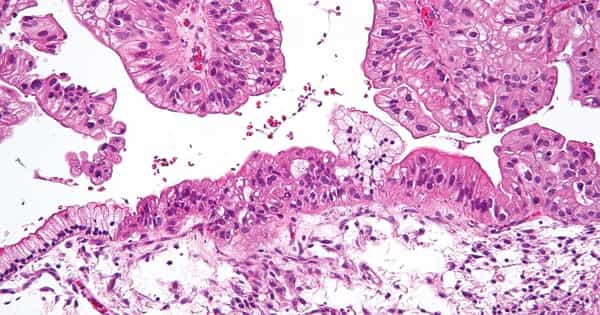
Ovarian cancer is one of the most dangerous neoplastic diseases that can affect women. It is made up of distinct types of carcinoma, each with its own clinicopathological and molecular characteristics. The Cancer Genome Atlas and other investigator-initiated genomic analyses have recently revealed the genome-wide molecular genetic landscape in various histological subtypes of ovarian cancer.
More than half of all ovarian clear cell carcinomas (OCCC) have mutations in the ARID1A gene, for which there are no effective treatments. The Wistar Institute scientists discovered that the loss of ARID1A function enhances a cellular stress response pathway that promotes cancer cell survival, making cancer cells more sensitive to pharmacological inhibition of this pathway. These findings, which were published online in Cancer Research, a journal of the American Association for Cancer Research, indicate a new therapeutic opportunity for this type of ovarian cancer, for which new treatments are desperately needed.
Inactivating mutations in the ARID1A tumor suppressor gene are the genetic drivers of OCCC, which does not respond to chemotherapy and has the worst prognosis of any ovarian cancer subtype.
Mutations in the ARID1A gene are present in more than 50% of ovarian clear cell carcinomas, for which effective treatments are lacking. Scientists discovered that loss of ARID1A function enhances a cellular stress response pathway that promotes survival of cancer cells.
“The goal of our research is to uncover the molecular changes caused by ARID1A loss so that we can specifically target them to achieve effective therapies for this devastating disease,” said Rugang Zhang, Ph.D., deputy director of The Wistar Institute Cancer Center, professor and program leader of the Immunology, Microenvironment, and Metastasis Program, and lead author of the study. “In this study, we focused on a stress response mechanism used by tumors to survive and discovered a link that provides a therapeutic opportunity.”
The endoplasmic reticulum (ER) is a cellular structure that regulates protein production and contains complex mechanisms to respond to stress caused by misfolded protein accumulation. Cancer cells frequently hyper-activated the ER stress response in order to survive in stressful microenvironment conditions. As a result, inhibiting this mechanism has been investigated as a therapeutic strategy for cancers with hyperactive ER stress responses.
Ovarian cancer is one of the gynecological malignancies with the poorest prognosis and the lowest overall and disease-free survival rates compared to other gynecological cancers. Ovarian tumors are classified into three types based on their cell of origin: epithelial, stromal, and germ cell tumors. Epithelial ovarian tumors have a high degree of histological heterogeneity and can be classified as benign, intermediate or borderline, or invasive.
The major signaling pathway involved in the ER stress response is the IRE1a/XBP1 pathway. The protein Inositol-required enzyme alpha (IRE1a) detects ER stress and activates the transcription factor X-box binding protein 1 (XBP1), resulting in the upregulation of genes involved in ER stress resolution and cell survival.
Zhang and colleagues discovered that ARID1A inhibits the IRE1a/XBP1 pathway and that loss of ARID1A in ovarian cancer causes increased activation of the pathway, leading to cancer cells’ reliance on upregulated IRE1a-XBP1. Indeed, treatment of ovarian cancer cells that do not express ARID1A with the selective IRE1a inhibitor B-I09 caused cell death as a result of unresolved ER stress.
“In some cases, the mechanisms that cancer cells use to their advantage make them vulnerable because they become dependent on specific pathways,” Zhang added. “If we can find ways to block those pathways, we might be able to use them as weak points in cancer-killing.”
This finding was supported in vivo, as B-I09 treatment reduced tumor burden and improved survival in mouse models with ARID1A-inactivated ovarian tumors. Cancer cells develop therapeutic resistance, allowing them to evade the effects of single-agent treatments, and combination strategies offer a solution to this major challenge. As a result, researchers combined IRE1a inhibition with histone deacetylase 6 (HDAC6) inhibition, which is also effective against ARID1A-mutant cancers. Because HDAC6 controls the degradation of misfolded proteins, the researchers hypothesized that the two treatments would work together to suppress ARID1A-mutant cancers.
“Our findings suggest that pharmacological inhibition of the IRE1a/XBP1 pathway of the ER stress response, alone or in combination with HDAC6 inhibition, represents a potential therapeutic strategy for ARID1A-mutated cancers,” said Joseph Zundell, a pre-doctoral researcher in the Zhang lab and the paper’s first author.