Intermolecular hydrogen bonding type of bond is formed between the two molecules of the same or different compounds. Some examples of the compounds exhibiting intermolecular hydrogen bonds are:
δ+ δ-
Hydrogen fluoride, H – F. In the solid state, hydrogen fluoride consists of long zig-zag chains of molecules associated by hydrogen bonds as shown below:
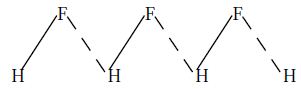
Therefore, hydrogen fluoride is represented as (HF)n.
Water: In the water molecule, the electronegative oxygen atom forms two polar covalent bonds with two hydrogen atoms. The oxygen atom due to its higher electronegativity acquires partial negative charge and the two hydrogen atoms acquire the partial positive charge. The negatively charged oxygen forms two hydrogen bonds with two positively charged hydrogen atoms of two neighbouring molecules. Each oxygen atom is tetrahedrally surrounded by four hydrogen atoms as shown below:
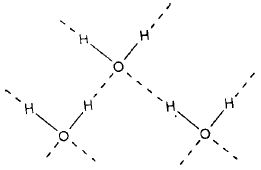
Hydrogen bonding in water results in a hydrogen bridge (H-O-H) network extending in three dimensions and the associated water molecule may be expressed as (H2O)n.