Electronegativity may be defined as the relative tendency of an atom in a molecule to attract the shared pair of electrons towards itself.
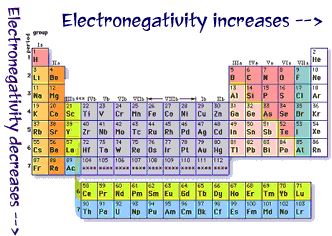
In a period, electronegativity increases on moving from left to right. This is due to the reason that the nuclear charge increases whereas atomic radius decreases. In a group, electronegativity decreases on moving down the group.
This is due to the effect of the increased atomic radius.
A few irregularities that are seen in the increasing values of ionization potential along a period can be explained on the basis of the concept of half-filled and completely filled orbitals.