Isomerism of Haloalkanes:
Haloalkanes are alkanes in which one or more of the hydrogen atoms has been replaced by a halogen atom. Haloalkanes show the following two types of isomerism.
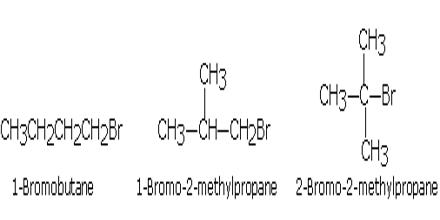
i) Chain isomerism: In this type of isomerism, the isomers differ in the chain in the carbon atoms. Haloalkanes containing four or more carbon atoms exhibit chain isomerism in which the isomers differ in the chain of carbon atoms. For example a halobutane (C4H9Br) has the following three chain isomers.
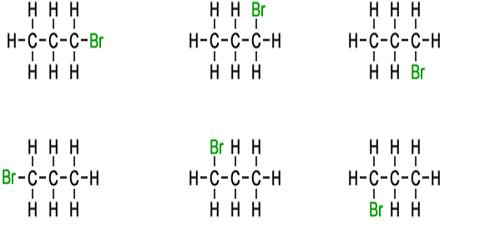
ii) Position isomerism: Position isomerism is due to different positions of the halogen atom in the chain. Haloalkanes with three or more carbon atoms exhibit position isomerism in which the isomers differ in the position of halogen atom. Halalkanes containing three or more carbon atoms show position isomerism for examples, halopropane (C3H7X) has two position isomers.