Chloroform (Formula: CHCl3) is a colorless, explosive, liquid plagiaristic of trichloromethane with an ether-like odor. It is a sweet smelling liquid having freezing point -63°C and has boiling point 61°C. It is the colorless non-flammable liquid that is insoluble in water. It is the simplest tri-halogen compound in which hydrogen atoms of methane are substituted by three chlorine atoms.
Laboratory Preparation of Chloroform from ethanol:
CHCl3 is prepared in the laboratory from ethanol or acetone.
Principle: Chloroform is prepared in the laboratory by the action of ethanol with a fine paste of bleaching powder in water. Bleaching powder supplies the chlorine and Ca(OH)2 necessary for a reaction; Cl2 acts as an oxidizing agent as well as chlorination agent and Ca(OH)2 is used as an alkali. Once made from acetone and bleaching powder, chloroform is now prepared by the photochemical reaction of methane with chlorine.
Chloroform is prepared in the laboratory by heating a combination of bleaching powder with ethanol or acetone. In this reaction, bleaching powder acts as an oxidizing chlorinating and hydrolyzing agent.
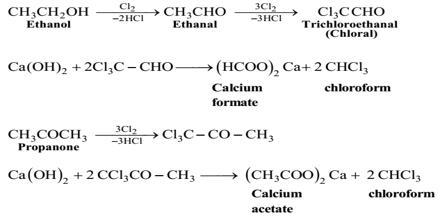
Step-1: At first bleaching powder and water (1:2) react to form chlorine and lime water [Ca(OH)2]
Ca(OCl) + H2O = Cl2 + Ca(OH)2
Step-2: (Oxidation): In this step, ethanol is oxidized by chlorine of bleaching powder to form ethanaldehyde.
CH3 – CH2 – OH + C12 = CH3 – CHO + 2HCl.
Step-3 (Chlorination): The produced ethanaldehyde reacts with chlorine to produce chloral.
CH3 – CHO + 3Cl2 = Cl3C – CHO + 3HCl.
Step-4 (Hydrolysis): In this step, the produced chloral undergoes hydrolysis with Ca(OH)2 with the formation of chloroform and calcium formate.
Cl3C – CHO + Ca(OH)2 = 2CHCl3 + (HCOO)2Ca
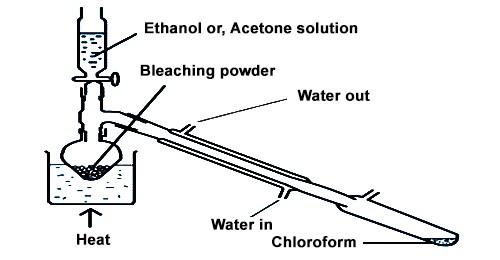
Procedure –
In the surrounding bottom flask, bleaching powder paste is arranged by adding 100g of bleaching powder to 200 ml of water and crushing the residue of bleaching powder in a mortar with the help of a pestle. After that 25 ml of ethanol or acetone is added to it. The flask is then fixed with a water condenser at the other end of which a receiver is joined. The flask is then placed over a sand bath and heated till a mixture of chloroform and water distills over. The mixture from the receiver is transferred to a separating funnel and the lower layer of chloroform is separated.
Purification –
Chloroform obtained by heating bleaching powder paste with acetone or ethanol is not in pure form. To take away impurities, it is conceded through dilute caustic soda solution and washed with water in an extrication funnel. The chloroform is then dried over anhydrous CaCl2 and redistilled between the temperature 60°C-65°C.
Uses of Chloroform –
Chloroform was formerly used as an inhaled anesthetic throughout surgery, the main use of chloroform today is in manufacturing, where it is used as a solvent and in the production of the refrigerant Freon. It is used for industrial purposes is generally made by the action of iron and acid on carbon tetrachloride. Chloroform is a sweet smelling liquid which is used for the following a purpose.
- It is used as the solvent for the production of dyes and pesticides.
- Chloroform is mostly used tools in kidnapping especially in books and movies.
- Chloroform usually contains heavy metal like deuterium which is used for solvent as Nuclear Magnetic Resonance Spectroscopy.