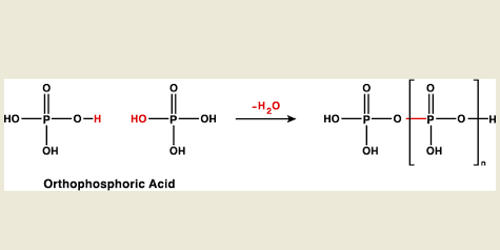
Preparation: Pyrophosphoric acid (H4 P2 O7) is prepared by heating orthophosphoric acid to 523 K – 533 K. This acid may be prepared by heating orthophosphoric acid at 215°C.
2H3PO4 → H4P2O7 + H2O
The acid solution in pure form can be obtained by ion exchange, passing an aqueous solution of sodium pyrophosphate, Na4P2O7, through a suitable cation exchange column.
This acid could be prepared by passing dry gaseous hydrogen chloride through crystals of disodium phosphate. An excess of hydrogen chloride could be removed by heating the mixture until the elimination of the hydrogen chloride is complete. Prolonged heating, particularly over 180° C, should be avoided as orthophosphoric acid dehydrates forming the pyrophosphoric acid (H4P2O7). In this manner, syrupy orthophosphoric acid in an amount corresponding with a yield of 75%, of the theoretical quantity is obtained.
The reaction mixture must be thoroughly stirred or agitated and when the reaction starts a considerable amount of heat is evaluated. When the reaction moderates, the liquid is gently heated, water is added to make up for the loss by evaporation. If pure calcium phosphate and hydrofluoric acid are used, the orthophosphoric acid obtained by the described method is very pure. During the heating and evaporation process, the temperature must not exceed 180° C.
Physical Properties
- It is a colorless crystalline solid.
- White to very pale yellow glassy solid.
- Crystallizes in two anhydrous forms: a metastable form melting at 54.3°C and a second and more stable form melting at 71.5°C.
- Extremely soluble in cold water, reacting very slowly to form phosphoric acid; decomposing much faster in hot water; very soluble in alcohol and ether.
Chemical Properties
- It is reconverted to orthophosphoric acid on boiling with water
H4P2O7 + H2O →2H3PO4
- When heated strongly, it yields metaphosphoric acid
H4P2O7 → 2ΗPO3 + H2O
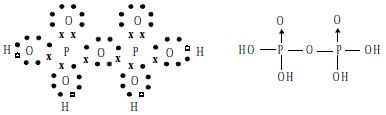
Structure: The Structure of pyrophosphoric acid is represented as:
Uses – Catalyst, manufacture of organic phosphate esters, metal treatment, stabilizer for organic peroxides.
Pyrophosphoric acid can be used for the preparation of vanadyl pyrophosphate methoxide cluster anion. This Acid is used in anion binding of inorganic phosphates due to its ability to form complexes.