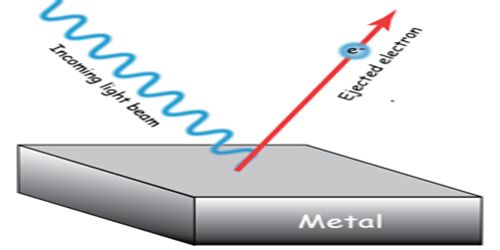
Laws of Photo Electric Emission
When the beam of the light enlightens the surface of the metals like sodium, potassium, cesium, and rubidium then their electrons start emitting. The phenomenon of emission of electrons is known as the photoelectric emission. In 1922, from the experimental results of Lennard, Thomson, Richardson, and Compton it has been established that photoelectric emission obeys the following laws.
First Law: Photoelectric emission is an instantaneous phenomenon. That means, if there is any time lag between the incidence of light and the emission of electrons, then that must be less than 3 x 10-9 second.
Second law: In case of each emission of photoelectron, there is a minimum frequency of the rays of incident light which is called the threshold frequency.
Third law: If the frequency of the incident light is greater than the threshold frequency then the photoelectric current is proportional to the intensity of light i.e., i ∞ I.
Here i = photoelectric current and I = intensity of light.
Fourth law: The velocity i.e., the kinetic energy of photo-electron does not depend on the intensity of the incident light; rather it depends on the frequency of the incident light and on the nature of the emitter.