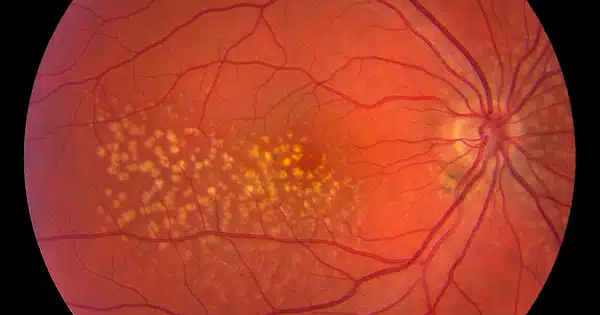
Retinal scans are a promising technology for tracking human aging in a non-invasive and low-cost manner. The retina is a thin layer of tissue at the back of the eye that detects light and sends visual information to the brain. It has blood vessels and other structures that can tell you about your overall health and well-being.
Is the eye a window into the aging process? According to new research, imaging of the fundus, the blood vessel-rich tissue in the retina, can be used to track human aging in a noninvasive, less expensive, and more accurate way than other aging clocks currently available. Researchers also conducted a genome-wide association study (GWAS) to determine the genetic basis for such a clock, dubbed eyeAge.
Pankaj Kapahi, a Buck Institute professor, believes the eye is a window into aging. His lab has demonstrated how imaging of the fundus, the blood vessel-rich tissue in the retina, can be used to track human aging in a way that is noninvasive, less expensive, and more accurate than other aging clocks currently available, in collaboration with Google Health and Zuckerberg San Francisco General Hospital. Researchers also conducted a genome-wide association study (GWAS) to determine the genetic basis for such a clock, which they call eyeAge, and published their findings in eLife.
Our study emphasizes the value of longitudinal data for analyzing accurate aging trajectories. Through EyePACS longitudinal dataset involving multiple scans from individual people over time our results show a more accurate positive prediction ratio for two consecutive visits of individual rather than random, time-matched individuals.
Sara Ahadi
“This type of imaging could be really valuable in tracking the efficacy of interventions aimed at slowing the aging process,” says Kapahi, a senior co-author of the study. “The results suggest that potentially, in less than one year we should be able to determine the trajectory of aging with 71% accuracy by noting discernable changes in the eyes of those being treated, providing an actionable evaluation of gero-protective therapeutics.” Kapahi noted that retinal scans are likely more reliable because changes in the eye are less susceptible to day-to-day fluctuations compared to biomarkers from the blood which are more dynamic and can be influenced by something as simple as eating a meal or a current infection.
A growing body of evidence suggests that the retina’s microvasculature may be a reliable indicator of the overall health of the body’s circulatory system and brain. Many age-related diseases, including age-related macular degeneration (AMD), diabetic retinopathy, and Parkinson’s and Alzheimer’s disease, cause changes in the eye. Ophthalmologists are frequently able to detect early signs of AIDS, chronic high blood pressure, and tumors in the eyes, which is not surprising given that any subtle changes in the vascular system first appear in the smallest blood vessels, and capillaries in the retina are among the smallest in the body.
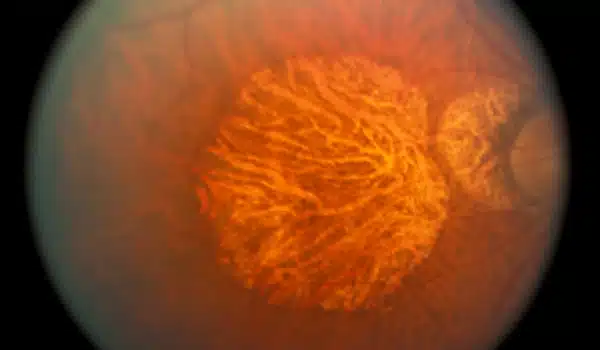
However, subtle changes in these small blood vessels frequently go undetected by even the most sophisticated instruments, necessitating the use of deep learning, a project led by Google Research. Researchers from Google and other companies created models to predict diabetic retinopathy from retinal images, and they have since used retinal images to identify at least 39 eye diseases, including glaucoma, diabetic retinopathy, and AMD, as well as non-eye diseases like chronic kidney disease and cardiovascular disease.
Google researchers trained and fine-tuned the model for eyeAge using their well-studied EyePACS data set, which includes over 100,000 patients, and then applied it to patients from the UK Biobank, which includes over 64,000 patients.
“Our study emphasizes the value of longitudinal data for analyzing accurate aging trajectories. Through EyePACS longitudinal dataset involving multiple scans from individual people over time our results show a more accurate positive prediction ratio for two consecutive visits of individual rather than random, time-matched individuals,” says Sara Ahadi, co-corresponding author and a former Fellow at Google Research who is now Senior Computational Biologist at Alkahest.
Noting that eyeAge is independent from phenotypic age (a well-established aging clock based on blood markers), Ahadi adds, “We are looking at aging through a different lens and bringing more information to the table. We hope eyeAge will be utilized along with other clocks to make tracking aging more robust, powerful and comprehensive.”
The GWAS was carried out at the Buck Institute using biological samples from the UK Biobank. Kenneth Wilson, a postdoctoral researcher at the Buck Institute, validated some of the genes identified in the study, building on previous Buck research that discovered a link between diet, eye health, and lifespan in Drosophila. Wilson discovered nearly 30 genes linked to visual decline, diabetes, hearing loss, Alzheimer’s disease, cardiovascular disease, and stroke in patient samples. ALKAL2, one of the genes, has previously been shown to increase lifespan in Drosophila (via the fly homolog ALK). Wilson knocked out the gene in the flies, which improved their vision and extended their lifespan.
According to Kapahi, the research findings are ready for further investigation. “It would be really interesting to understand how these genes, which have already been linked to other age-related diseases, are influencing the changes we’re seeing in the eye,” he says. “This is human data that can be used to target potential treatments for age-related diseases.” The possibility of tracking their efficacy in such a low-cost, non-invasive manner is a huge plus.”