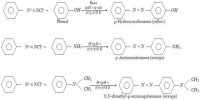
Below Describe Melting Point and Boiling point of Alkanes, Alkenes and Alkynes
Melting Point and Boiling point of Alkanes
- Branched isomer has less melting point and boiling point than their corresponding simple isomer because simple isomer has more Van dar walls force than branched isomer.
- The melting point and boiling point of alkanes show a gradual increase with the increase in the molecular mass.
Melting Point and Boiling point of Alkenes
- Simple isomer has more melting point and boiling point than the corresponding branched isomer.
- The melting point and boiling point increases with the increase in the molecular mass.
Melting Point and Boiling point of Alkynes
- Alkynes have melting point and boiling point than their corresponding Alkanes and Alkenes.
- The melting point and boiling point increases with the increasing molecular mass.