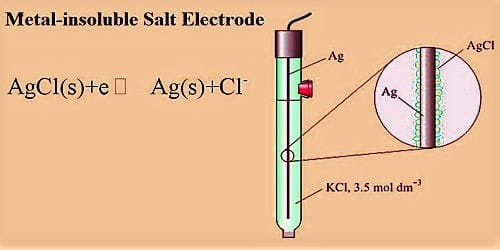
A metal-insoluble salt electrode in Half-Cells
A half cell is one of the two electrodes in a galvanic cell or simple battery. For example, in the Zn-Cu battery, the two half cells make an oxidizing-reducing couple. The cation part in the electrolyte must have standard reduction potential less than the standard reduction potential of the cation of the metal salt.
This consists of a metal in contact with an insoluble salt of the metal which, in turn, is in contact with a solution containing the anion of the insoluble salt. A more elaborate but generally acceptable and regularly used electrode consists of a metal in contact with an insoluble salt of the metal, which in turn in contact with a solution containing the anion of the salt. A typical electrode of this kind consists of a silver wire covered with a thin coating of silver chloride, which is insoluble in water. Frequently used electrode consists of a metal in contact with an insoluble salt of the metal, which in turn in contact with a solution containing the anion of the salt. An example is the silver, silver chloride electrode represented as-
Ag, AgCl (s)│Cl– (C1)
The electrode reaction can be considered as taking place in mo steps:
Ag (s) ↔ Ag+ (aq) + e–
Ag+ (aq) + Cl– (aq) ↔ AgCl (s)
—————————————-
Ag (s) + Cl– (aq) ↔ AgCl (s) + e–
The electrode reaction involves only the concentration of Cl–; the electrode is said to be reversible with respect to Cl–. The above reaction shows how an electron can be released or taken up in such an electrode. Another example of a metal insoluble salt electrode is the mercury, mercurous sulfate electrode Hg, Hg2SO4(s)│SO42+ (c) used in the standard Weston cell.
The metal/insoluble salt electrode usually consists of a metal electrode in contact with (partially coated with a thin layer of) an insoluble salt of the metal. This congregation is then held in contact with a solution containing the anion of the insoluble salt. Electron transfer occurs between the metal atoms of the electrode and the metal ions in the insoluble salt. These electrodes are constructed for any metal with its insoluble metal salt dipped in a solution containing the common anion of the salt and not the common cation (obviously the anion in the solution should come from highly soluble electrolyte).
The most frequently used electrode of this type is the calomel electrode, which consists of mercury in contact with mercurous chloride (calomel) as a paste, over which is placed a solution containing chloride ions, usually KCl. The electrode which is represented as Hg, Hg2Cl2(s) / C– (c) is shown in Figure. The electrode is usually made with 0.1 mol L-1 or saturated KCl solution. A paste of mercury and calomel is first made by thorough grinding. This is then washed several times with small portions of the KCl solution. The paste is placed in the clean electrode vessel in which some amount of pure mercury had already been placed. The rest of the vessel is then filled with the KC1 solution. A tube at the end of which a piece of platinum wire is sealed is placed in the vessel as shown in Figure.
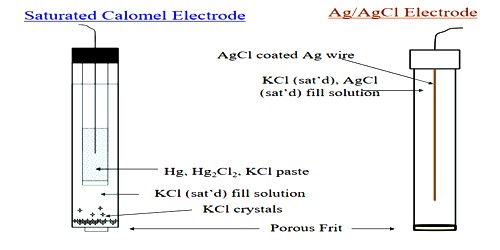
Some mercury is placed in this tube. Contact with mercury in the vessel is made by dipping a thick copper wire in the mercury in the tube. Examples of this type of half cell, when used as a cathode, are Cu2+ | Cu, Zn2+ | Zn, Ag+ | Ag, Sn2+ | Sn, etc. The half cells or electrodes used in Daniel cell are of the metal-metal ion type.
In the electrochemical cell, the electrode having higher oxidation potential undergoes oxidation and acts as the anode or negative electrode and the electrode having lower oxidation potential undergoes reduction and acts as the cathode or positive electrode. Moreover, the cation in the solution should have a standard reduction potential less than that of the electrode under consideration.