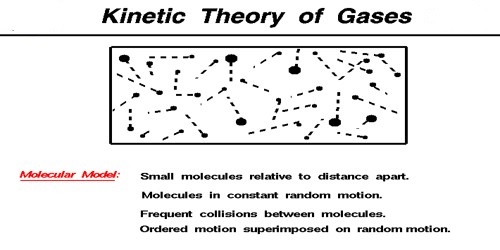
Molecular Kinetic Theory of Gases
We know that solids, liquids and gases are composed of innumerable tiny particles. These particles are called molecules of the material. Actually, the smallest particles of matter which can freely exist and in which all the properties of matter exist is called molecule of matter. Molecules of the same matter are identical; on the other hand molecules of different matters are different.
Molecules of matter do not remain in contact with each other; there exists a very small gap between them. This gap or separation is called inter-molecular separation or gap. Inspite of this gap molecule, assert force on each other. This is called inter-molecular force. From the difference in physical properties of solids, liquids and gases nature of inter-molecular force can assessed.
In case of gases inter-molecular gap as quite large. No force is needed to separate any part of a gas. So, even a small amount gas in a container occupies total volume of the container. In reality, intermolecular force between molecules of gases is very small. It is clear from this discussion that as the inter-molecular gap increases inter-molecular attraction rapidly decreases. Again, if the gap decreases attractive force increases rapidly.
According to this theory molecules are always in motion. Molecules move from one place to another place or continue to vibrate from a point. This motion of the molecules appears as heat energy.
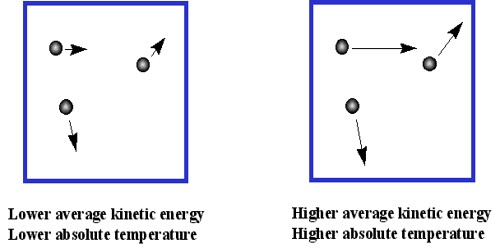
According to kinetic theory temperature is the main source of kinetic energy of gas molecules. Average kinetic energy of gas molecules is proportional to absolute temperature (E = 3/2 KT). Since there is no attraction or repulsion between the molecules, so their potential energy is zero.
Molecules have kinetic energy only and temperature is decided by that kinetic energy only. So it can be said- “Temperature is the external demonstration of kinetic energy.” Concept on which kinetic theory of gases is based is that molecules are in random motion with high speed because of thermal agitation. During this motion they collide with each other and also with walls of the container. Hence gas has no fixed shape or volume. It takes the shape of the container it is kept in. During the middle of nineteenth century Maxwell, Boltzmann, Leans, Regnault, Van der Waals and other scientists gave molecular concept of kinetic theory.