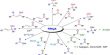
n-type Semiconductor
Pentavalent elements are mixed with germanium or silicon semiconductor to produce n-type semiconductor. Pentavalent antimony or arsenic is mixed at high temperature by a special process. During mixing the amount of impurity is controlled in such a way as the main structure of germanium or silicon is not changed, rather their atoms are incorporated in the crystal lattice. Arsenic or antimony contains 5 valence electrons. 4 electrons in its outermost shell are shared with 4 electrons in neighboring germanium or silicon. Thus 4 covalent bonds are formed. In each atom of arsenic or antimony, one electron remains excess and that electron can move freely with the crystal [Figure].
![]()
Fig: n-type Semiconductor
So, it is seen that each impurity atom gives one free electron. Hence the impurity atom is called a donor atom. Besides, due to thermal agitation, some equal number of electrons and holes are produced. An n-type semiconductor is made by adding a small amount of a Group-V element such as phosphorous (P) or arsenic (As) to the intrinsic semiconductor. Group-V elements have five valence electrons per atom. So, in an n-type semiconductor, both electrons and holes are present. But a number of electrons are manifolding greater than that of holes. Crystal produced in this way contains about 1017 number of free electrons. In electric conduction negative electrons play the main role, hence these are called ‘majority carriers’.
Positive holes play a minor role in electric conduction; hence these are called ‘minority carriers’. When we use a pentavalent impurity for doping then we get an n-type semiconductor. Examples of pentavalent impurities are phosphorus or arsenic.