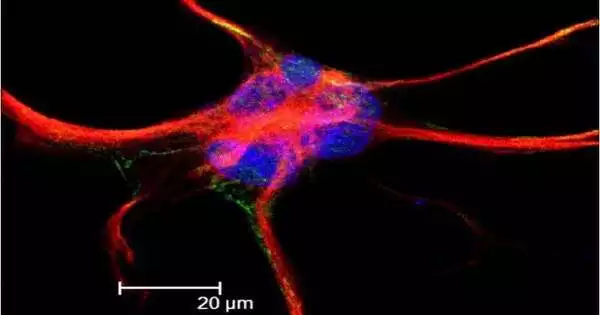
Neuronal activity has a significant impact on the development and function of astrocytes. Astrocytes are a type of glial cell in the brain that works closely with neurons to support their development, function, and survival. Neuronal activity has been shown in studies to regulate astrocyte proliferation, differentiation, and morphology. For example, neural activity patterns influence astrocyte proliferation and differentiation during development. Neurons produce factors that promote astrocyte differentiation, and astrocytes, in turn, help neurons survive and function.
According to the researchers, neuronal activity is both necessary and sufficient for astrocytes to develop their complex shape, and disrupting this developmental process disrupts brain function.
Baylor College of Medicine researchers have uncovered the processes that give astrocytes, the most abundant glial cell in the brain, their distinctive bushy shape, which is critical for brain function. They report in Nature that neuronal activity is both required and sufficient for astrocytes to develop their complex shape, and that disrupting this developmental process results in disrupted brain function.
This finding was surprising and very interesting. Neurotransmitters, such as GABA, are known to signal between neurons at synapses, but we discovered that neurotransmitters also signal astrocytes, influencing their development by triggering changes in their structure.
Dr. Benjamin Deneen
“Astrocytes play a variety of important roles in brain function,” said first author Yi-Ting Cheng, a graduate student in Dr. Benjamin Deneen’s lab at Baylor. “For example, they promote the activity of other essential brain cells, neurons; they participate in the formation and function of synapses, or neuron-to-neuron connections; they release neurotransmitters, chemicals that mediate neuronal communication; and they form the blood-brain barrier.”
The bushy shape of astrocytes in the adult brain is fundamentally linked to effective brain function. The branched-out astrocyte structure interacts with neurons and controls synaptic activity. “If astrocytes lose their structure, then synapses do not behave properly and brain function goes awry,” said Deneen, professor and Dr. Russell J. and Marian K. Blattner Chair in the Department of Neurosurgery and director of the Center for Cancer Neuroscience at Baylor. He also is the corresponding author of the work.
“Figuring out how astrocytes acquire their complex, bushy structure is essential to understanding how the brain develops and functions and may bring new insight into how neurodevelopmental conditions emerge. In this study, we investigated the cells and processes that direct the development of astrocyte structure.”
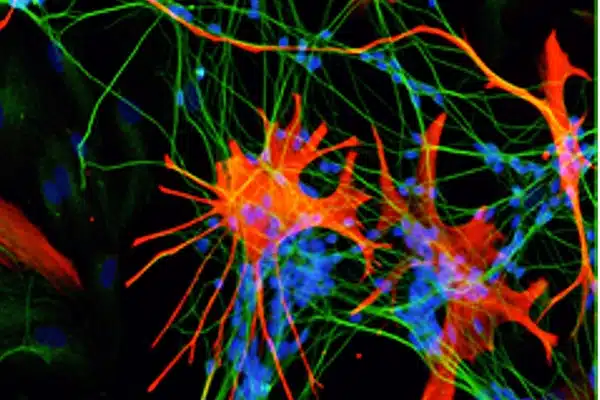
Neurons lead the way
When astrocytes develop, neurons are already present and active, so do neurons influence how astrocytes acquire their complex shape?
“We artificially activated or silenced neurons and determined whether this would speed up or slow down astrocyte maturation,” Cheng said. “We found that neuronal activity is both necessary and sufficient to drive full astrocyte maturation into a bushy-shaped cell.”
So, how do astrocytes receive the signals that guide them down the correct maturation path? The team discovered that neurons produce a neurotransmitter called GABA, which binds to astrocytes via a molecule on their surface known as the GABAB receptor. “We activated neurons by knocking out the GABAB receptor in astrocytes.” The neurons did not promote the development of a typical astrocyte shape in this situation, lending credence to the idea that neurons communicate with astrocytes via the GABAB receptor to promote their maturation.”
“This finding was surprising and very interesting,” Deneen said. “Neurotransmitters, such as GABA, are known to signal between neurons at synapses, but we discovered that neurotransmitters also signal astrocytes, influencing their development by triggering changes in their structure.”
Other experiments added to the puzzle of how neurons cause astrocytes to develop their bushy shape. “GABA is produced by neurons and binds to astrocytes via the GABAB receptor.” As a result, a series of events occur, including the activation of another receptor called Ednrb, which drives pathways that remodel cellular architecture inside the cells associated with cell shape,” Cheng explained.
Another mystery concerning astrocyte development was also investigated by the researchers. They discovered that the expression of GABAB receptor in astrocytes is not regulated in the same way in different brain regions. “This outcome was completely unexpected,” Deneen said. “The GABAB receptor is universally required for astrocytes to develop their bushy shape in all brain regions.” “How is it regulated differently in different areas of the brain?”
The researchers discovered that this regional regulation is conferred by two proteins, LHX2 in the brain cortex and NPAS3 in the olfactory bulb, via region-specific interactions with proteins SOX9 and NFIA, which are present in all astrocytes and regulate GABAB receptor expression.