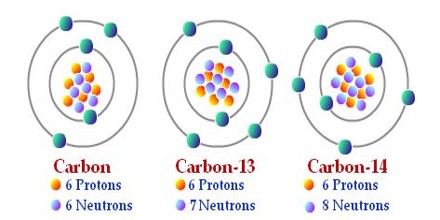
Isotopes are the variants of a definite element. Atoms of the same element having different mass number are called isotopes. That is, in isotopes of an element. the number of protons is same but the number of neutron is different. The number of protons in the nucleus of atom of an element identifies the element uniquely. But in principle, an element may have any number of neutrons. The total number of protons and neutrons present in the nucleus of an element is its mass number. For this reason, each isotope of an element has different mass number. Carbon can be considered as an example. Three isotopes of carbon 12C6, 13C6 and 14C6 – the mass number of them are 12, 13, 14 respectively. The atomic number of carbon is 6, i.e. there are six protons in each carbon atom. As a result, the number of neutrons in the isotopes of carbon is 6, 7 and 8 respectively.
Uses of Isotopes
In the field of medical science, radioactive isotopes are widely used in nuclear medicine. Radioisotopes have two types of applications.
- For diagnosis purpose
- For treatment purpose
The presence of harmful cancer tumor anywhere in the body or in an organ can be identified by radioisotopes. The energetic gamma rays emitted from the isotope Co-60 is used for the treatment of cancer. The gamma rays emitted from Co-60 is used to sterilize surgical instruments. Iodine-131 (131I) is used for the treatment of the abnormal growth of the thyroid gland. Technetium-99m is the most widely used radioactive isotope for diagnostic studies in nuclear medicine. Technetium is used for brain, bone, liver and spleen imaging or scanning. Blood- Leucaemia caused by excess of white blood cell is treated with phosphate of radioactive phosphorus-32. In nuclear medicine, radioisotopes are introduced into the body of the patient through the veins to diagnose diseases. The radioactive materials are selected depending on the organ which will be examined. Besides these, radioisotopes are widely used in the field of agriculture, food preservation, controlling pests and industries.