Photoelectric Work Function: The amount of energy needed to emit the electron from the attraction of the nucleus of the emitter is called the photoelectric work function. It is defined as the minimum amount of energy required by an electron to escape from a metal surface. The minimum energy needed to eject an electron from a metal is known as the work function of the metal. The work function is different for different metals. The minimum energy required to eject an electron from the surface is called the photoelectric work function.
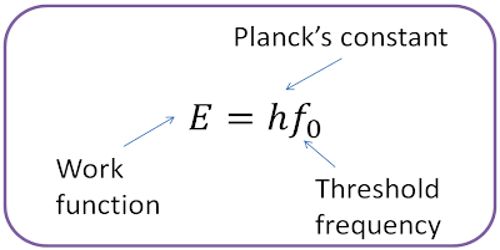
The minimum amount of energy which is necessary to start photoelectric emission is called Work Function. If the amount of energy of incident radiation is less than the work function of the metal, no photoelectrons are emitted.
It is denoted by Φ0. The work function of a material is given by Φ0 = hv0.
It is a property of the material. Different materials have different values of work function. Generally, elements with low I.P values have a low work function such as Li, Na, K, Rb, and Cs.