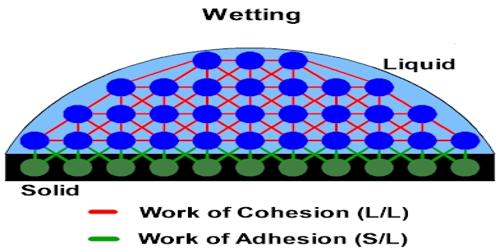
Cohesive force: We know a certain material consists of some molecules. The attractive force acting between molecules of the same materials is called cohesive force. It is the force binding the molecules of the same substance, like the cohesive force between the water molecules however adhesive force exists between two different substances.
These attractive forces exist between molecules of the same substance. For instance, rain falls in droplets, rather than a fine mist, because water has strong cohesion which pulls its molecules tightly together, forming droplets. Cohesion holds hydrogen bonds together to create surface tension on water. Since water is attracted to other molecules, adhesive forces pull the water toward other molecules. Cohesive forces are attractive forces between similar molecules. Ex- between water-water molecules.
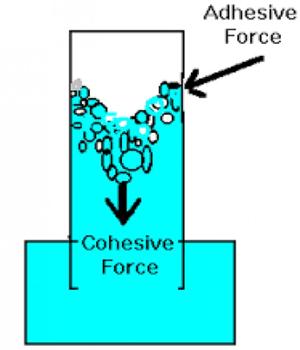
For example, the attractive force between molecules of iron is the Cohesive force. This force obeys the inverse square law of distance.