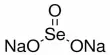
ETH Zurich researchers have developed a cell culture test to detect substances that are directly or indirectly harmful to embryos. The augmented version, which is based on an existing test used for developing new drugs and chemicals, is intended to help reduce the number of animal experiments.
Drugs must be safe not only for the patients but also for the unborn children in the womb in the case of pregnant patients. As a result, candidate substances are tested in the Petri dish on embryonic stem cells from mouse cell lines at an early stage in the development of new medicines. This is done to avoid the possibility of an embryo-damaging effect being discovered later on during tests with pregnant mice.
These cell culture tests, however, are a highly simplified version of what happens in the uterus. Researchers can identify substances that have a direct negative effect on embryonic stem cells by simply adding the test material to a culture of embryonic stem cells in a Petri dish. Active pharmaceutical ingredients, on the other hand, may be modified by the mother’s metabolism and enter the embryo’s bloodstream via the placenta in the body of a pregnant woman. Furthermore, standard cell culture tests cannot detect substances that have indirect effects on the embryo, such as interfering with placental function or causing stress responses.
Researchers developed a cell culture test to detect substances that are directly or indirectly harmful to embryos. A Swiss team has created a laboratory test that incorporates the placenta into embryotoxicity assessments without damaging fetuses.
A chip with different cell types
Researchers at ETH Zurich in Basel’s Department of Biosystems Science and Engineering have developed a laboratory test that incorporates the role of the placenta in embryotoxicity assessments. Julia Boos, a doctoral student in the group of ETH Professor Andreas Hierlemann, and her colleagues created a new chip to accomplish this. This chip has several compartments that are linked together by miniature channels.
On this chip, the researchers combined human placental cells derived from cell lines with microtissue spheroids derived from mouse embryonic stem cell lines, known as “embryoid bodies,” which represent the embryo’s early development. The test substances must first pass through a layer of placental cells before reaching the embryonic cells, simulating the situation in utero.
These experiments, by the way, do not result in viable embryos. Over a ten-day period, embryonic cells derived from cell lines only go through the very first stages of embryonic development.
Test detects indirect damage
The researchers used microparticles that did not harm the embryoid bodies when they came into direct contact with them to demonstrate how the new test worked. However, with the new test, which includes placental cells, the researchers discovered a possible indirect negative effect. Although the placental cells were able to keep the microparticles at bay, ensuring that the particles did not reach the embryonic cells, the placental cells displayed a detectable stress response.
The researchers would now like to improve their system by using better plastic materials. In the future, human stem cell lines could be used instead of mouse cells to form embryoid bodies. “There are significant differences between lab animals and humans, particularly in terms of embryonic development and placental processes,” Boos says, adding, “Of all the organs, the placenta is where differences between the species are most pronounced.”
The group’s goal is to develop a new test that is also simple to use in the pharmaceutical industry. The ability to detect—and eliminate—substances that are harmful to the embryo at an early stage of drug development means that fewer substances will be tested on animals in in-vivo studies later on.
Testing for toxic effects on the placenta can be done at various levels, which affects not only the quality and quantity of information but also the costs and workload. The system not only allows for the assessment of substance transfer across the placental barrier, as previously described, but placental cells and tissues can also be used as models for investigating toxic effects on human tissue.