Antihistamines have been linked to improved immunotherapy responses, according to researchers. Their research revealed that the histamine receptor plays a role in suppressing T cell activation and thus blocking anti-tumor immune responses.
Treatment with antihistamines, a common allergy medication, was associated with improved responses to immune checkpoint inhibitors, according to new research from The University of Texas MD Anderson Cancer Center. The preclinical study found that the histamine receptor H1 (HRH1) suppresses T cell activation in the tumor microenvironment in tumor-associated macrophages (TAMs). The findings were published in Cancer Cell.
If replicated in prospective clinical trials, the findings suggest that targeting HRH1 in combination with checkpoint blockade may be useful in overcoming immunotherapy resistance and improving outcomes, particularly in patients with pre-existing allergies or high plasma histamine levels.
“We were surprised to discover that antihistamines, a mediator of the allergy response, were associated with significantly improved outcomes in patients while searching for factors that might influence responses to immunotherapy,” said study co-lead Yi Xiao, Ph.D., instructor of Molecular & Cellular Oncology. “We discovered that histamine, via its receptor HRH1, can promote cancer cell immune evasion and resistance to immunotherapy.”
We were surprised to discover that antihistamines, a mediator of the allergy response, were associated with significantly improved outcomes in patients while searching for factors that might influence responses to immunotherapy.
Yi Xiao
Antihistamines associated with improved immunotherapy outcomes
Immune checkpoint inhibitors, a type of immunotherapy, work by inhibiting specific checkpoint proteins that regulate T cell activity, allowing T cells to mount an anti-tumor response and eliminate cancer cells. Many patients benefit from checkpoint blockade, but not everyone benefits equally. As a result, there is a desire to learn more about the factors that influence immunotherapy sensitivity or resistance.
The researchers began this study by looking into whether other commonly used medications could influence responses to checkpoint inhibitors. They conducted a retrospective analysis of clinical data from MD Anderson patients receiving immune checkpoint inhibitor treatment.
Concurrent use of antihistamines targeting HRH1 was associated with significantly improved survival outcomes in patients with melanoma or lung cancer. Patients with breast or colon cancer showed similar trends, but the data did not reach statistical significance due to the small sample size.
Using The Cancer Genome Atlas and other publicly available patient cancer data, the researchers discovered that high HRH1 expression in tumors was associated with markers of T cell dysfunction, poor responses to checkpoint inhibitors, and worse survival outcomes.
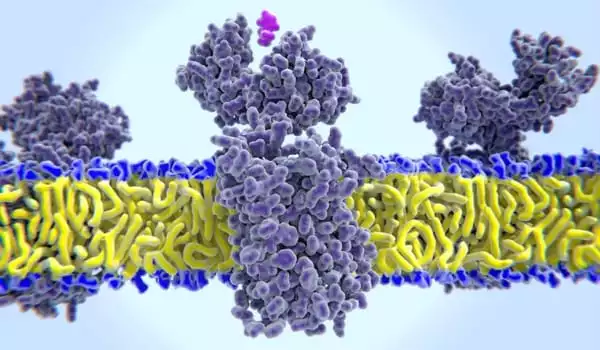
Histamine receptor acts in tumor microenvironment to suppress T cell activation
Following the observed correlations, the researchers sought to clarify the potential contributions of HRH1 and its ligand, histamine, to the immune response.
Both proteins were found to be elevated in the tumor microenvironment, but they did not appear to be from the same source. HRH1 was not found in cancer cells, but it was found in high concentrations in certain types of TAMs in the tumor microenvironment known as M2-like macrophages, which aid in immune suppression. Cancer cells, on the other hand, appear to be a major source of elevated histamine levels in patient samples and cancer cell lines.
In preclinical models, blocking HRH1 on macrophages (via genetic knockout or antihistamine treatment) reduced TAM immune-suppressive activity, resulting in increased T cell activation and tumor growth inhibition.
The researchers examined additional regulatory receptors on macrophages to better understand how HRH1 in TAMs influences T cell activity. By inhibiting HRH1 activity, the membrane localization of VISTA, an inhibitory receptor known to suppress T cell activation, was reduced. Furthermore, inhibiting HRH1 caused widespread changes in gene expression, resulting in a shift from M2-like characteristics to a more pro-inflammatory state consistent with M1-like macrophages.
HRH1 acts in TAMs to drive cells into an immune-suppressive M2-like state and to increase membrane expression of the inhibitory checkpoint VISTA, ultimately leading to dysfunctional T cells and a suppressed anti-tumor response.
Targeting HRH1 enhances checkpoint blockade responses in preclinical models
Combining an antihistamine with checkpoint blockade improved therapeutic efficacy and prolonged survival in preclinical models of breast cancer and melanoma. Furthermore, in preclinical models, the antihistamine produced similar results as treatment with anti-VISTA antibodies, which is currently being evaluated in clinical trials.
Furthermore, the researchers investigated the effects on tumor progression using a preclinical model of allergic disease. Histamine levels and tumor growth increased after allergies were induced compared to controls. These effects, however, could be reversed with antihistamine treatment.
Similarly, the researchers discovered a link between plasma histamine levels in cancer patients and responses to immune checkpoint inhibitors. These findings imply that increased histamine levels, whether caused by allergies or cancer cell production, may contribute to the suppression of the anti-tumor response.
“Our preclinical findings suggest that antihistamines have the potential to enhance immunotherapy responses, particularly in those with high blood levels of histamine,” said corresponding author Dihua Yu, M.D., Ph.D., chair ad interim of Molecular & Cellular Oncology. “There is still work to be done, but we are excited to continue investigating potential therapeutic applications for antihistamines, which offer a low-cost approach with few side effects.”
Moving forward, the team is developing prospective clinical trials to assess the combination of antihistamines and checkpoint inhibitors in cancer patients.