Specialized type of unsaturation: Aromatic compounds are unsaturated cyclic compounds.
- Aromatic compounds take part in addition reaction with H2, X2, O2 under drastic condition but not with hydro-halogen acids (HX)
- They are very stable and are not oxidized with KMnO4 solution and not affected by acids.
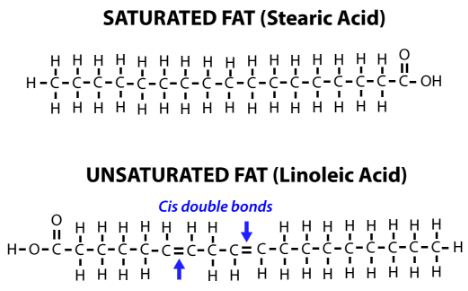
- The double bond between two carbons prevents rotation of the atoms about the bond, locking them into specific structural formations.
- Unsaturated compounds are those in which addition reaction can be obtained. In a chain of carbons, such as a fatty acid, a double or triple bond will cause a kink in the chain.