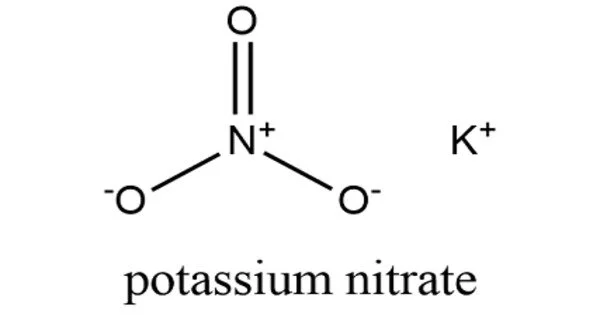
Potassium nitrate (KNO3) is a chemical compound with the formula KNO3. It has the appearance of a white to dirty gray crystalline solid. It is an alkali metal nitrate because it is an ionic salt of potassium ions K+ and nitrate ions NO3−. It serves as a fertilizer. It is found in nature as a mineral called niter (or nitre in the UK). It is a nitrogen source, and nitrogen was named after niter. Potassium nitrate is one of several nitrogen-containing compounds known as saltpetre.
Potassium nitrate is widely used in fertilizers, tree stump removal, rocket propellants, and fireworks. It is a significant component of gunpowder (blackpowder). Potassium nitrate reacts with hemoglobin and myoglobin in processed meats, producing a red color.
Properties
At room temperature, potassium nitrate has an orthorhombic crystal structure that changes to a trigonal system at 129 °C (264 °F). It is slightly soluble in water, but its solubility increases with increasing temperature. The aqueous solution is almost neutral, with a pH of 6.2 at 14 °C (57 °F) for a 10% commercial powder solution. It is not very hygroscopic, absorbing only about 0.03 percent of the water in an environment with an average relative humidity of 80% over 50 days. It is not poisonous and is insoluble in alcohol; it can react explosively with reducing agents but is not explosive on its own.
- Chemical formula: KNO3
- Molar mass: 101.1032 g/mol
- Appearance: white solid
- Odor: odorless
- Density: 2.109 g/cm3 (16 °C)
- Melting point: 334 °C (633 °F; 607 K)
- Boiling point: 400 °C (752 °F; 673 K) (decomposes)
- Solubility in water: 133 g/1000 g water (0 °C); 2439 g/1000 g water (100 °C)
- Solubility: slightly soluble in ethanol; soluble in glycerol, ammonia

Production
It occurs naturally as niter in rocks in India, South Africa, and Brazil. When heated, it decomposes into nitrite and oxygen. It is not deliquescent, unlike sodium nitrate. Potassium nitrate is used in gunpowder, fertilizers, and the laboratory preparation of nitric acid.
Potassium nitrate can be made by combining ammonium nitrate and potassium hydroxide.
NH4NO3 (aq) + KOH (aq) → NH3 (g) + KNO3 (aq) + H2O (l)
Combining ammonium nitrate, found in instant ice packs, and potassium chloride, easily obtained as a sodium-free salt substitute, is an alternative method of producing potassium nitrate without a byproduct of ammonia.
NH4NO3 (aq) + KCl (aq) → NH4Cl (aq) + KNO3 (aq)
Potassium nitrate can also be produced by neutralizing nitric acid with potassium hydroxide. This reaction is highly exothermic.
KOH (aq) + HNO3 → KNO3 (aq) + H2O (l)
On industrial scale it is prepared by the double displacement reaction between sodium nitrate and potassium chloride.
NaNO3 (aq) + KCl (aq) → NaCl (aq) + KNO3 (aq)
Uses
Potassium nitrate has a wide variety of uses, largely as a source of nitrate.
- Nitric acid production
Historically, sulfuric acid was combined with nitrates such as saltpeter to produce nitric acid. In modern times, this is reversed: nitrates are made from nitric acid, which is produced through the Ostwald process.
- Oxidizer
The most well-known application of potassium nitrate is as an oxidizer in blackpowder. From the beginning of time until the late 1880s, blackpowder provided explosive power for all firearms on the planet.
Meat processing
Since antiquity or the Middle Ages, potassium nitrate has been a common ingredient in salted meat. The widespread use of nitrates is more recent, and it is associated with the development of large-scale meat processing. Potassium nitrate use has largely been discontinued due to slow and inconsistent results when compared to sodium nitrite compounds such as “Prague powder” or pink “curing salt.”