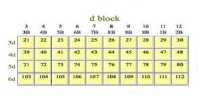
Properties of Zinc
Physical properties:
i) Zinc is a bluish white metal
ii) It is good conductor of heat and electricity.
iii) It is malleable and ductile.
Chemical properties
i) Action of air
When heated in air at 773 K, it burns to form a white cloud of Zinc oxide which settles to form a wooly flock called philosopher’s wool.
- 2Zn + O2 ⎯⎯⎯773K→ 2ZnO
ii) Action of water
Pure zinc does not react with water but impure zinc (Zn-Cu couple) decomposes steam quite readily evolving H2 gas.
- Zn + H2O (steam) → ZnO + H2
iii) Action of dilute acids
Pure zinc is not attacked by dilute acids. However, impure zinc reacts with dilute acids with the liberation of H2.
- Zn + 2HCl → ZnCl2 + H2↑
- Zn + H2SO4 → ZnSO4 + H2↑
iv) Action of con.H2SO4
Zinc reacts with hot con.H2SO4 forming ZnSO4.
- Zn + 2H2SO4 → ZnSO4 + SO2 ↑ + 2H2O
v) Action of HNO3
Zinc reacts with HNO3 at various concentrations and it gives different products.
- 4 Zn + 10 HNO3 (dil) →4 Zn (NO3)2 + N2O + 5 H2O (Nitrous oxide)