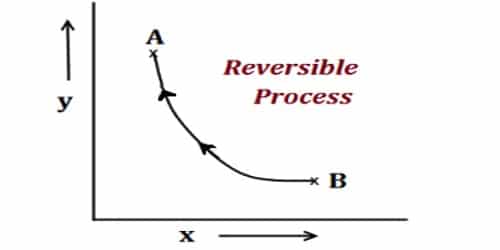
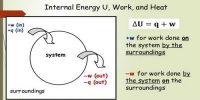
The process in which the system and surroundings can be restored to the initial state from the final state without producing any changes in the thermodynamic properties of the universe is called a reversible process. The progression in which the arrangement and surroundings can be restored to the primary state from the last state without producing any changes in the thermodynamic properties of the universe is called a reversible process. Usually, it happens when that system is close to thermal equilibrium. This equilibrium has to be surrounded by the system itself and also within the system and its surroundings.
Reversible processes are differentially removed from symmetry with no (appreciable) internal temperature, pressure, and velocity changes.
Real-Life Examples of Reversible Process
At normal pressure and at 273 K temperature if some amount of ice absorbs heat for transformation into the water and equal amount of water rejects the same amount of heat in order to be transformed into an ace, then it is a reversible process.
Some examples of reversible prose are given below. In reality, it is difficult to give an example of the fully reversible process. But there are some processes which may apparently be considered as reversible. Some of these processes are mentioned below:
(i) If isothermal and adiabatic processes are executed very slowly then they will be reversible. This is because of that as there is no dissipative force like friction and since the process is done very slowly so heat or energy is not dissipated due to conduction, convection, and radiation.
(ii) In warm season we make water to get freeze into ice cubes. Upon use of these ice cubes, they again get converted into water. At normal pressure and at 00C temperature, by absorbing 80 calories or 336 J of heat each gm of ice is transformed into water. Again at normal pressure and at 00C temperatures by rejecting 80 calorie or 336 J of heat each gm of water is transformed into ice. So, the process is reversible.
(iii) In laboratories, water can be made which the help of hydrogen and oxygen atoms. And this water can again be transformed into divide hydrogen and oxygen atoms and the procedure is called hydrolysis.
(iv) If an elastic ball is dropped from a stroll height on an elastic steel sheet, as there is no loss of energy, the ball will again rise to the initial height. So the process is reversible.
(v) If a spring is expanded very slowly within elastic limit the amount of work done on the spring in each step, the spring will do the same amount of work during compression. So, the process is reversible.
(vi) Plants make food from nutrients it collects from the soil with the help of roots. Nutrients are transformed into complex food like starch. We eat plants and our body breaks this complex food again into simple nutrients which can be used by the body. That is from simple to complex than complex to simple.
(vii)One which can be considered is Nitrogen taken from the soil by plants is again returned to the soil during decomposition of plants when it is dead.