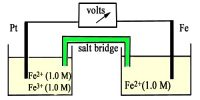
A cell electromotive force (or “emf”) is a measure of the driving force of the electro-chemical cell reaction. The reaction at the anode has a definite oxidation potential, while the reaction at the cathode has a definite reduction potential.
Thus, the overall cell emf is a combination of these two potentials and therefore we have Eau = oxidation potential + reduction potential.
A voltage cannot be measured just for a half-cell but only for a complete cell. Therefore, to describe the reduction potential for a particular redox couple, the emf is measured for a cell where the other half-cell is a standard reference electrode.
The reference electrode is usually assigned a potential of zero and the potential of the other electrode is obtained relative to this by measuring the cell emf. Typically, the reference chosen is the standard hydrogen electrode.
Remember measurements are made under standard conditions
- Pressure of any gas: 1 atmosphere
- Concentration of solutions: 1 mol.dm-1
- Temperature: 298 K (25°C)
For example, for a cell composed of a zinc electrode connected to a hydrogen electrode, the measured emf is 0.76 V. Zinc is found to act as the anode, so the Zn/Zn+2 oxidation potential is 0.76 V.
Standard electrode potentials are usually all listed as reduction potentials i.e. by reversing the oxidation potential. Thus, the Zn/Zn+2 reduction potential is listed as EZn = – 0.76V.