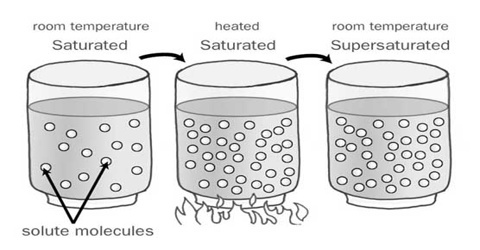
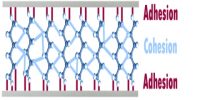
A Supersaturated solution describes a solution that has dissolved more than the maximum amount of solute, reached by heating then cooling. There are some substances which can form solutions that are termed supersaturated as these can contain more of the solute than is required to make a saturated solution. A saturated solution of sodium acetate at 00C contains 119 g of the solute in 100 g of water. It is more soluble at higher temperature. If an unsaturated hot solution of sodium acetate containing more than 119 g of solute, say 125 g, in 100 g of water is cooled slowly to 0°C the excess solute remains dissolved: the solution is no supersaturated. The supersaturated solution is in unstable equilibrium as the introduction of a small crystal or a little jerk will Start crystallization until the excess solid is separated.
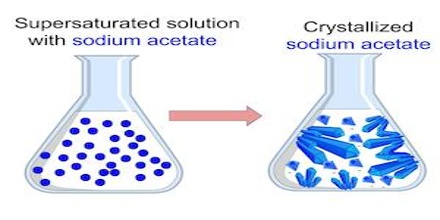
A supersaturated solution is when you pour the solute into the solvent and the solute doesn’t dissolve. For example, if you have cup of iced tea, and you pour sugar into the tea, it is supersaturated when the sugar settles on the bottom of the glass. If you were to take the ice out of the tea, the sugar could then dissolve.