In an attempt on enliven the idea of Democritus, John Dalton; an English scientist produced his idea about the identity and properties of atoms and their ability to form compounds, combining with each other. It is known as Dalton’s Atomic Theory. The main postulates of the Theory are discussed below:
i) Matter is composed of small particles called Atoms. The smallest unit of an element is called Simple Atom. While that of compound is called a Compound Atom.
ii) The mass and properties shown by all the atoms of an element are always same.
iii) Atoms cannot be divided into smaller pieces. They take part in reactions remaining intact.
iv) Atoms are neither created nor destroyed in chemical reaction. They can only change from one form to another.
v) In the formation of a certain compound the atom always combine in a constant ratio of their number.
vi) Elements can combine in different ratio to form different compounds.
Limitations of Dalton’s Atomic Theory:
Dalton’s Atomic theory was the first to provide scientific ideas relating to the structure of matter and this was the greatest success that his theory had achieved. Moreover the theory also explains the process of the chemical reactions. Still there were limitations for instance,
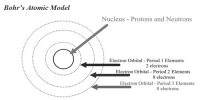
i) According to Dalton’s Atomic theory Atom is indivisible but afterwards it is established that Atoms can be divided into smaller particles like electron, proton, neutron etc.
ii) Dalton proposed that all the atoms in an element have the same mass which has been proved wrong. This is because isotope has different mass.
iii) Similarly when isobars were discovered, it was found that atoms of different elements can have the same mass. This goes against the Dalton’s theory.
iv) According to Dalton the smallest unit of a compound is the compound atom which is against our knowledge of molecule. Thus Dalton’s theory would not distinguish between atoms and molecule.