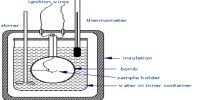
It is important to give the exact reaction equation when quoting the associated energy change. 2Mg (s) + O2 (g) → 2MgO (s), ΔH = – 1204 kJ mol-1. For example, if no reaction equation was stated, ΔH for this reaction is -1204 kJ mol-1 of O2, but only 602 kJ mol-1 of Mg.
Always give the states of the reactants and products when quoting H for a reaction.
E.g. Pb (s) + Cl2 (g) → PbCl2 (s), H= – 359 kJ mol-1 There would be a large extra energy change needed to change Pb (s) into Pb (g)!
Rules:
- If a certain process has an enthalpy change DH, the reverse of that process has an enthalpy change of -ΔH. Example: H2O (s) → H2 O (l) ΔH = 6.00 kJ
- Multiplying a thermochemical equation by a constant also multiplies the thermodynamic quantity by that constant.
- The thermodynamic quantity for the reaction applies as the equation is written. So, it can be used as a stoichiometric ratio with any of the reactants or products in the reaction. For, example, in the reaction: H2 (g) + Cl2 (g) → 2 HCl (g) ΔHº = -183 kJ