Electrons can either be core electrons or valence electrons. The valence electrons are the outermost electrons of an atom.
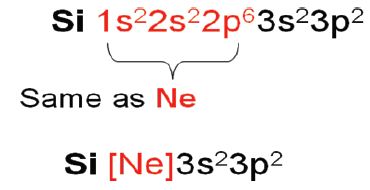
As orbitals are filled, core electrons are part of the inner electronic configuration. This can be used to write electronic configuration in short hand notation.
Valence electrons
Valence electrons occupy the highest energy (outer) shelL They are the electrons most affected by the approach of another atom. They are the electrons most likely to be involved when bonding occurs.
The number of valence electrons dictates the way an element reacts, so the Periodic Table relates electronic configuration to chemical properties. Elements with the same number of electrons in the outer shell will have similar chemical properties, and are arranged underneath one another in Groups. The number of electrons permitted in each atomic orbital underlies the layout of the Periodic Table. The 7 horizontal rows on the Periodic Table correspond to the main energy levels. Each row (called a Period) represents a value of n – the Principal Quantum Number.
As each successive element adds one more electron, the Aufbau principle means that the energy sub-levels will be filled in order of increasing energy. Thus for each new value of n, the first two elements will add electrons that will fill the s orbital. These are the elements in Group 1 & 2 – the “s block”. Then the next six elements will each add an extra electron into the p orbitals. These are the elements in Groups 13 – 18 – the “p block”. From the n = 4 Period, the order of the energy levels (shown by the Aufbau Principle) has to be studied carefully. After the 4s sub-level, the 3d orbital is filled next. This holds up to 10 electrons. These elements are the first row of Transition Metals – the “d block”. Eventually, the f orbitals are filled as well – these elements in the “f block” are sometimes called the Inner Transition Metals.











