If a raisin is kept in a cup of water for some times it will be shown that the raisin will absorb water and become full and flashed. The outer covering of the raisin is a semi permeable membrane. Out side this membrane there is water in the cup, and inside the membrane there is sweet juice of raisin. The amount of water in the cup is more (actually whole amount is water) but that inside the raisin is very little. In this condition water from the cup enter into the concentrated region inside through the semi permeable membrane of raisin. As a result the raisin swollen and filled with juice. It is happened by a special process known as Osmosis.
Osimosis may be defined as -a process by which solvent (water) diffuse from an area of high concentration through a semi permeable membrane to a area of low concentration solution is termed as Osmosis. The process continues till the concentration of two solutions become same. Osmosis is a special type of diffusion.
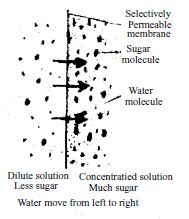
We know solution is a mixture of solute and solvent, for example mixture of sugar and water is a solution. Here water is the solvent and sugar is the solute and the mixture is the solution. In osmosis diffusion of solute never takes place, only solvent diffuses that is in case of plants only water diffuses.
Two types of Osmosis:
* Endosmosis: osmosis in which solvent (water) from outside enter inside the cell, is called endosmosis. Concentration of cell sap is more than the concentration of the outer solution. Put some raisins in water Water will enter inside the fruits (raisin) and the raisin will swell up like a grape being filled with juice.
* Exosmosis: Osmosis in which water (solvent) comes out from inside the cell is called exosmosis. Exosmosis takes place when the concentration of external solution is more than the concentration of cell sap.
Put some grape in salt solution and observe it. Water will come out from the grape and it will contract.