Primary Standard Substance
The substances which do not react with the components of air (O2, CO2, etc.) in open state and retained its composition for a long time is called primary standard substance. e.g. Na2CO3, K2Cr2O7, etc. are the primary standard substance.
A primary standard is a reagent that is enormously pure, constant, has no waters of hydration, and has a high molecular weight. Standards are materials containing a known concentration of a substance. They provide a reference to determine unknown concentrations or to calibrate analytical instruments.
Some important examples of the primary standard are;
- Sodium Carbonate (Na2CO3)
- Potassium hydrogen phthalate (KHP) (KHC8H4O4)
- Potassium dichromate (K2Cr2O7)
In chemistry, a primary standard is a consistent, readily eligible substance. Some features of a primary standard are;
- High purity
- Stability (low reactivity)
- Low hygroscopicity and efflorescence
- High solubility (if used in titration)
- High equivalent weight
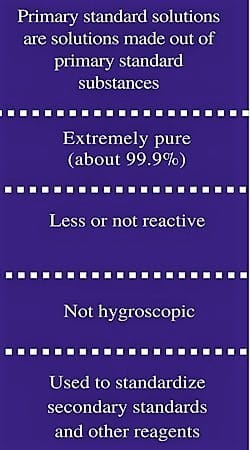
Fig: Common features of a primary standard
Primary Standard Solutions
In chemistry, the term “primary standard” refers to a compound the chemist uses to find out the concentration of another compound or solution. For example, you can’t make certain the concentration of a solution of sodium hydroxide (NaOH) by simply dividing the mass of NaOH by the volume of its solution.
- Stable in Air
A primary standard cannot decompose in, absorb or otherwise react with any components of air. Many iron (II)-based compounds, for example, react with oxygen in the air to become iron (III) compounds. Primary standards also cannot absorb water or other distinctive components.
- Soluble in Water
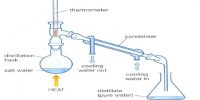
Chemists almost always carry out reactions involving primary standards in aqueous solutions, which require that the primary standard melt simply in water. Silver chloride (AgCl), for example, satisfies all of the other requirements of primary standards, but it will not dissolve in water and consequently cannot serve as a primary standard.
- Highly Pure
Any impurity in a primary standard result in inaccuracy in any measurement that involves its use. Primary standard reagents naturally exhibit purities of 99.98 percent or greater. Chemists use silver nitrate (AgNO3), for example, as a primary standard, but not all samples of silver nitrate possess the essential purity for this application.
- High Molar Mass
Compounds of high molar mass or molecular weight necessitate comparatively large sample masses for the chemist to carry out the consistency reaction on a reasonable scale. Weighing out large samples reduces the inaccuracy in the mass measurement.
Primary standards have several unusual properties –
- They are no sensitive to atmospheric oxygen
- They have known method and molecular weight
- They are generally high molecular weight compounds
- They have a stable concentration/uniform composition for a long period of time
- They are dominant reactants.