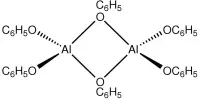
Aluminum fluoride is a chemical compound with the chemical formula AlF3. It is a white solid that is highly soluble in water and is commonly used in the production of aluminum metal and as a flux in the production of high-purity aluminum. They are all colorless solids. Anhydrous AlF3 is used in the production of aluminium metal.
AlF3 is used in the manufacturing of catalysts, enamels, and pharmaceuticals. It should be noted that AlF3 can be highly toxic if inhaled, and can cause respiratory irritation and lung damage. Therefore, it is important to handle AlF3 with caution and in accordance with proper safety protocols.
Properties
- Chemical formula: AlF3
- Molar mass: 83.977 g/mol (anhydrous), 101.992 g/mol (monohydrate), 138.023 (trihydrate)
- Appearance: white, crystalline solid odorless
- Density: 3.10 g/cm3 (anhydrous), 2.17 g/cm3 (monohydrate), 1.914 g/cm3 (trihydrate)
- Melting point: 1,290 °C (2,350 °F; 1,560 K) (anhydrous) (sublimes)
- Solubility in water: 5.6 g/L (0 °C), 17.2 g/L (100 °C)

Production
Aside from anhydrous AlF3, several hydrates are known. With the formula AlF3·xH2O, these compounds include monohydrate (x = 1), two polymorphs of the trihydrate (x = 3), a hexahydrate (x = 6), and a nonahydrate (x = 9).
The majority of aluminium fluoride is produced by treating alumina with hydrogen fluoride at 700 °C: Hexafluorosilicic acid may also be used make aluminum fluoride.
H2SiF6 + Al2O3 + 3 H2O → 2 AlF3 + SiO2 + 4 H2O
Alternatively, it is manufactured by the thermal decomposition of ammonium hexafluoroaluminate. For small-scale laboratory preparations, AlF3 can also be prepared by treating aluminium hydroxide or aluminium metal with hydrogen fluoride.
Application
In addition to its industrial uses, aluminum fluoride has a number of important applications in the field of chemistry, including its use as a Lewis acid in organic syntheses and as a reagent in the production of other aluminum compounds. It is also used in some specialized applications, such as in the production of high-temperature superconductors and in the purification of semiconductors.
Safety
It is important to note that aluminum fluoride is highly toxic and should be handled with caution. Prolonged exposure to aluminum fluoride can cause respiratory and skin irritation, as well as other health effects.
Aluminum fluoride has a reported oral animal lethal dose (LD50) of 0.1 g/kg. Repeated or prolonged inhalation exposure can cause asthma, as well as effects on the bone and nervous system, resulting in bone changes (fluorosis) and nervous system impairment.
The formation of aluminum fluoride complexes, which mimic the chemical structure of phosphate and influence the activity of ATP phosphohydrolases and phospholipase D, is responsible for many of fluoride’s neurotoxic effects. Aluminum fluoride is formed using only micromolar concentrations of aluminum.