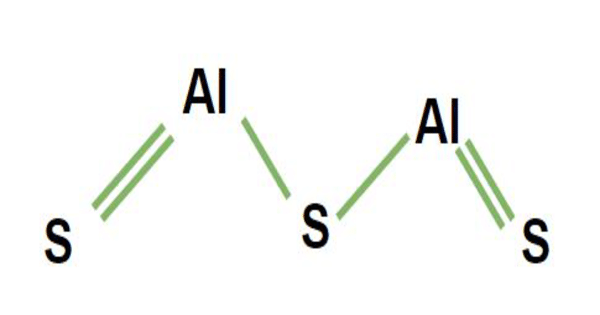
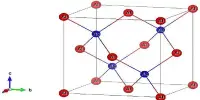
Aluminium sulfide is a chemical compound with the formula Al2S3. It is a white or gray crystalline powder that is insoluble in water. This colorless species has an intriguing structural chemistry and exists in several forms. The material is moisture sensitive, hydrolyzing to hydrated aluminum oxides/hydroxides. This can happen when the sulfide is exposed to air. The hydrolysis reaction produces gaseous hydrogen sulfide (H2S).
Aluminium sulfide is primarily used in the production of aluminum and in the manufacturing of chemicals such as sulfuric acid and aluminum sulfate. It is also used as a coagulating agent in water treatment and as a catalyst in organic synthesis.
Properties
- Chemical formula: Al2S3
- Molar mass: 150.158 g/mol
- Appearance: gray solid
- Density: 2.02 g/cm3
- Melting point: 1,100 °C (2,010 °F; 1,370 K)
- Boiling point: 1,500 °C (2,730 °F; 1,770 K) sublimes
- Solubility in water: decomposes
- Solubility: insoluble in acetone
- Crystal structure: trigonal

Preparation
Aluminium sulfide can be prepared by reacting aluminum with sulfur at high temperature, typically above 700°C. Aluminum sulfide is readily prepared by ignition of the elements
2 Al + 3 S → Al2S3
This reaction is extremely exothermic and it is not necessary or desirable to heat the whole mass of the sulfur-aluminum mixture; (except possibly for very small amounts of reactants). The product will be created in a fused form; it reaches a temperature greater than 1100 °C and may melt its way through steel. The cooled product is very hard.
Aluminium sulfide is a highly reactive compound and should be handled with care. It can react violently with water, releasing toxic hydrogen sulfide gas, and should not be stored in damp areas or exposed to moisture.
Applications
Aluminium sulfide is used in a variety of applications, including as a reducing agent in organic synthesis and as a coagulant in water treatment. It is also used in the production of other aluminium compounds and in the manufacturing of batteries. Additionally, it has been investigated for its potential as a semiconductor material.
Aluminium sulfide has some potential health hazards associated with it. Inhaling the dust or fumes from the compound can cause irritation to the respiratory tract, eyes, and skin. It may also have carcinogenic properties, although this has not been fully established. Therefore, appropriate safety precautions should be taken when handling this compound.