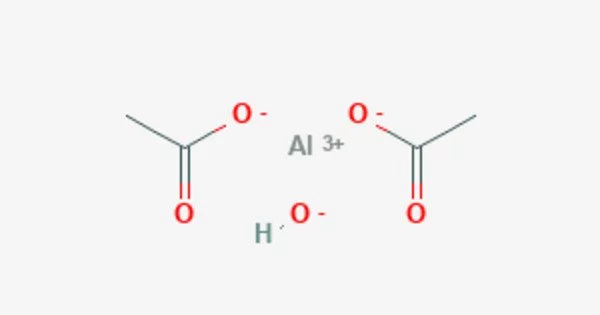
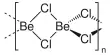
Aluminium triacetate is a white or colorless crystalline solid that is soluble in water and ethanol. It’s a chemical compound with the formula Al(CH3CO2)3. It appears as a white, water-soluble solid under standard conditions and decomposes when heated to around 200 °C. The triacetate hydrolyzes to a mixture of basic hydroxide / acetate salts, and multiple species co-exist in chemical equilibrium, particularly in aqueous solutions of the acetate ion; this mixed system is commonly referred to as aluminium acetate.
Aluminium triacetate is used both alone and in combination as a mordant agent with dyes such as alizarin. It is used with cotton, other cellulose fibers, and silk in conjunction with aluminium diacetate or aluminium sulfacetate. It has also been colored by combining it with ferrous acetate.
Properties
- Appearance: It is a white crystalline solid.
- Solubility: It is soluble in water and organic solvents such as ethanol.
- Stability: It is stable under normal conditions but decomposes at high temperatures.
- Reactivity: It is a weak Lewis acid and can react with Lewis bases to form coordination complexes.
Application
Aluminium triacetate is used in the production of textiles, paper, and leather. It is also used as a mordant in dyeing and printing processes. It is used as a coagulating agent in water treatment, particularly for the removal of organic matter from drinking water. It can also be used as a mordant in the textile industry, as a reagent in organic synthesis, and in the production of aluminum-containing chemicals.
Aluminium triacetate is considered to be relatively non-toxic. However, ingestion or inhalation of large amounts can cause irritation to the respiratory system and digestive tract. It is important to handle with care as it can be harmful if ingested, inhaled or comes in contact with the skin. It should be stored in a cool, dry place, away from incompatible materials such as strong oxidizing agents and acids.