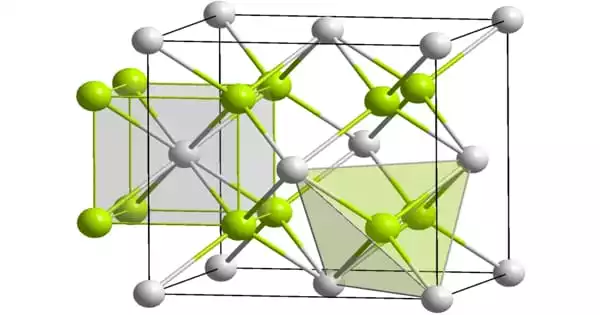
Americium dioxide (AmO2) is a dark americium chemical. In its solid state, AmO2 has the fluorite, CaF2 structure. It’s a source of alpha particles.
Americium occurs naturally in uranium minerals, but only in trace levels. The principal source of the element is the neutron bombardment of plutonium in nuclear reactors. Each year, a few grams are created in this manner.
Properties
- Chemical formula: AmO2
- Molar mass: 275 g·mol−1
- Appearance: Black crystals
- Density: 11.68 g/cm3
Historical context
The demand for americium dioxide stems from the difficulty of storing the element americium as a solution of americium(III) chloride because of the alpha radiation and hydrochloric acid decomposes storage containers over time. To solve the liquid storage problem, scientists at Oak Ridge National Laboratory devised a synthesis to turn liquid americium-acid solution into a precipitated form of americium for safer handling and more efficient storage.

Synthesis
The Oak Ridge National Laboratory’s 1960 description of americium dioxide synthesis begins with dissolving americium in hydrochloric acid and then neutralizing the excess acid with ammonium hydroxide (NH4OH). Then, a saturated oxalic acid solution is added to the now-neutralized solution to precipitate dull pink americium(III) oxalate crystals; once complete precipitation is accomplished, additional oxalic acid is added to form a slurry. The americium oxalate and oxalic acid slurry is then stirred before the americium oxalate is filtered out, rinsed with water, and partially dried in air.
After that, the americium oxalate is calcined in a platinum boat. It is dried in a furnace at 150°C before being heated to 350°C. When breakdown begins, the oxalate will decompose into the desired black americium dioxide; to guarantee no oxalate remains in the newly formed dioxide, the oven temperature is increased and held at 800 °C before slowly cooling to ambient temperature.
Modern applications
The most common americium chemical used in ionising smoke detectors is americium dioxide. Because the dioxide form is insoluble in water, it is reasonably safe to handle in the manufacturing process.
ESA became interested in americium dioxide as a power source for radioisotope thermoelectric generators (RTGs) for deep space research spacecraft and satellites in the late 2010s. Nuclear experts at the University of Bristol invented a completely automated chemical process to produce americium dioxide, which will be applied at the Sellafield nuclear plant in Cumbria, UK. It is founded on the same principles as the historic manufacturing method pioneered at Oak Ridge National Laboratory.
Health Hazards
Americium is a highly radioactive element that, if handled incorrectly, can be harmful and cause severe illnesses. Because it does not occur naturally in the environment, humans and animals are unlikely to be impacted by it unless they are in extremely close proximity to plutonium-based nuclear reactors.