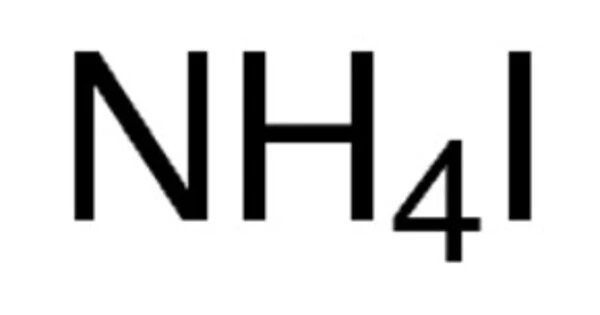
Ammonium iodide is an inorganic chemical having the formula NH4I. It is a white, crystalline substance that is very soluble in water. It is an ionic substance, and impure samples appear yellow. This salt is composed of an ammonium cation and an iodide anion. It can be produced by the action of hydroiodic acid on ammonia. It is highly soluble in water and crystallizes into cubes. It’s also soluble in ethanol.
Ammonium iodide can react with strong oxidizers and acids. When exposed to powerful oxidizing chemicals, it can be oxidized to produce nitrogen gas, water, and iodine.
Properties
- Physical Appearance: It typically appears as a white crystalline solid. It may also occur as a colorless to white powder.
- Solubility: It is highly soluble in water, meaning it readily dissolves in aqueous solutions.
- Odor: It has a slight ammonia odor due to the presence of the ammonium ion.
- Hygroscopic: Like many ammonium salts, it tends to absorb moisture from the air, making it somewhat hygroscopic.
- Melting Point: The melting point is around 551 degrees Celsius (1023.8 degrees Fahrenheit).
- Stability: It is stable under normal conditions, but it can decompose upon heating to release ammonia gas and iodine vapor.
Preparation
Ammonium iodide can be made in lab by treating ammonia with hydroiodic acid:
NH3 + HI → NH4I
Organic reactions are not manufactured using organic solvents due to their growing negative impact on the human body and the environment. Many chemists have modified organic processes to remove solvents in order to achieve good sustained synthesis. A study was made on an organic synthesis for the iodination of ketones and aromatic compounds utilizing ammonium iodide and hydrogen peroxide. This resulted in higher yields of the products, which were collected more efficiently and in a shorter period of time than when the abrasive component, molecular iodine, was used.
Uses
Ammonium iodide is used as dietary supplement to treat iodine deficiency. It is commonly used in photographic chemicals, in the manufacturing of dyes and pigments, and as a source of iodine in organic synthesis. Additionally, it has been used in some pharmaceuticals and as a reagent in analytical chemistry. However, it’s worth noting that due to its potential toxicity and environmental concerns, its usage has decreased over time, and safer alternatives are often preferred.
Toxicity and Hazards
It is generally considered toxic if ingested, inhaled, or absorbed through the skin. It can release ammonia gas upon heating, which can be irritating to the respiratory system.
Like many iodide compounds, prolonged exposure to high concentrations of ammonium iodide can lead to iodism, which includes symptoms such as skin rash, metallic taste, sore teeth and gums, headache, and gastrointestinal disturbances. However, acute toxicity from incidental exposure is relatively low.