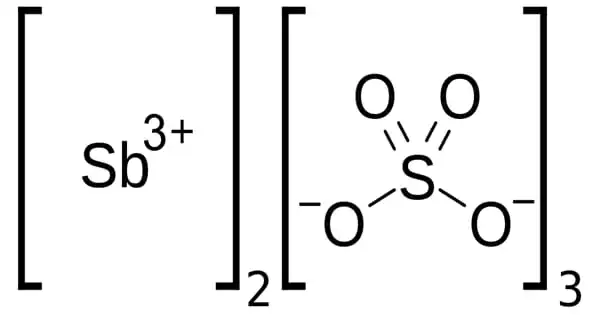
Antimony sulfate Sb2(SO4)3, is a hygroscopic salt produced by combining antimony or its compounds with hot sulfuric acid. It is a white, crystalline, deliquescent, water-insoluble material that is primarily employed in the production of explosives. It is used in semiconductor doping as well as the manufacture of explosives and fireworks.
Antimony is used as a reference electrode, as an electrocatalyst for electrochemical energy conversion, and as an inhibitor of iron and steel corrosion. Antimony is also utilized as an alloying ingredient in lead alloys used to cast lead-acid battery grids. It has a considerable impact not only on the casting and mechanical properties of the alloys, but also on the electrochemical processes of the battery’s positive plate.
Structure
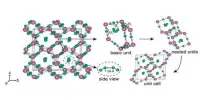
Solid antimony sulfate contains infinite ladders of SO4 tetrahedra and SbO3 pyramids sharing corners. It is often described as a mixed oxide, Sb2O3.3SO3.
Properties
- Chemical formula: Sb2(SO4)3
- Molar mass: 531.7078 g/mol
- Density: 3.6246 g/cm3
- Solubility in water: soluble

Chemical properties
Antimony sulfate is sometimes referred to as a “salt” because it can be produced by the reaction of antimony and sulfuric acid; however, antimony does not form a nitrate when dissolved in nitric acid (an oxidising acid), but rather a mixture of antimony oxides, in contrast to bismuth, which dissolves in both acids to form salts. It is delectable and acid soluble. Antimony, antimony trioxide, antimony trisulfide, or antimony oxychloride can be made by dissolving them in hot, concentrated sulfuric acid.
2 Sb (s) + 6 H2SO4 → Sb2(SO4)3 + 3SO2 + 6 H2O
Uses
Owing to its solubility, antimony sulfate has uses in the doping of semiconductors. It is also used for coating anodes in electrolysis and in the production of explosives and fireworks.
Extremely pure antimony is in high demand. Antimony oxide is becoming more used as a flame retardant. Antimony is a component of several lead and tin alloys, which are used to make bearings and solders. The preparation, characteristics, and applications of antimony halides, oxides, sulfides, and other compounds, as well as organometallic compounds, are discussed.
Safety
Antimony(III) sulfate causes irritation to the skin and mucous membranes.
Natural occurrence
The specific compound’s natural analogue is still unknown. Basic hydrated Sb sulfates, on the other hand, are known as the minerals klebelsbergite and coquandite.
Pyrometallurgical procedures are used largely to extract antimony metal from ore. Because rich ores are becoming scarce, more reliance is placed on intermediates in the processing and metal sectors. The Sunshine Mining Co. uses hydrometallurgical processing for some ores containing precious metals (USA). Pure antimony metal is created through refining and fine purification procedures.