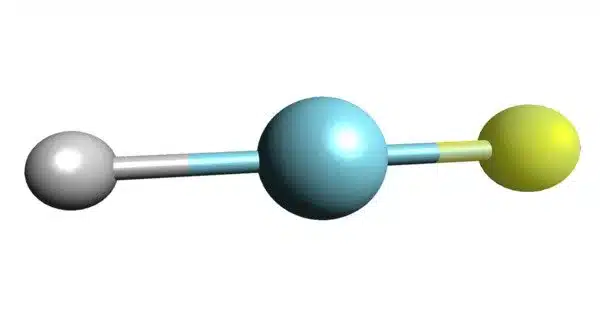
Argon fluorohydride or argon hydrofluoride is an inorganic compound with the chemical formula HArF (also written ArHF). It is a chemical compound consisting of argon, hydrogen, and fluorine. It is a compound of the chemical element argon. Its chemical formula is HArF, and it is a colorless gas at standard conditions.
AHF is an intriguing compound because it is highly unstable and reactive, and it can exist only at extremely low temperatures and pressures. Its instability stems from a very weak bond between the argon and fluorine atoms, which is easily broken by even a small amount of energy. Overall, AHF is an important compound in the study of noble gas chemistry, and it has potential applications in fields such as materials science, electronics, and laser technology.
Properties
- Chemical formula: HArF
- Molar mass: 59.954 g/mol
- Appearance: Unknown
- Density: Unknown
- Melting point: −256 °C (−428.8 °F; 17.1 K) (decomposes)
- Solubility in water: Unknown
Discovery
AHF is a very rare compound, and it was first synthesized in 2000 by a team of researchers at the University of Helsinki in Finland. It is considered to be one of the noble gas compounds, which are compounds that contain one of the noble gases (helium, neon, argon, krypton, xenon, or radon) as a constituent.
This argon compound was discovered by a group of Finnish scientists led by Markku Räsänen. They published their discovery of argon fluorohydride in the journal Nature on August 24, 2000. Although not the first, this discovery led to the recognition that argon could form weakly bound compounds.
Synthesis
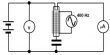
This substance was created by combining argon and hydrogen fluoride on a caesium iodide surface at 8 K (265 °C) and then exposing the mixture to ultraviolet radiation. As a result, the gases merged.
The infrared spectrum of the resulting gas mixture reveals the presence of chemical bonds, albeit very weak ones; thus, it is argon fluorohydride, not a supermolecule or a mixture of argon and hydrogen fluoride. Its chemical bonds are only stable when kept below 27 K (246 °C); when heated, it decomposes into argon and hydrogen fluoride.
Application
ArF is mainly used in excimer lasers, which are used in semiconductor manufacturing, eye surgery, and microlithography. ArF excimer lasers emit light at a wavelength of 193 nm, which is used to etch patterns on silicon wafers and other materials used in the manufacture of computer chips.
ArF is a highly reactive and toxic gas and must be handled with care. It can cause severe irritation to the eyes, skin, and respiratory system and can be lethal in high concentrations. As a result, it is typically used and handled only by trained professionals in controlled environments.