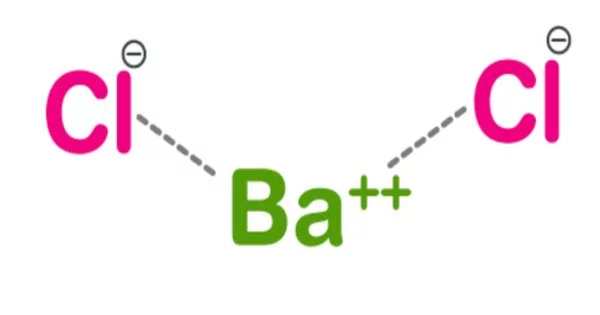
Barium chloride is an inorganic compound with the formula BaCl2. It is one of the most common barium salts that are water soluble. It is white, highly toxic, and imparts a yellow-green color to a flame, like most other water-soluble barium salts. It is also hygroscopic, converting first to the dihydrate BaCl2(H2O)2. It has limited application in the laboratory and industry.
It is a white solid chemical compound that is soluble in water, hygroscopic, and produces a faint yellow-green color when exposed to flame. Barium salts are widely used in industry. Sulfate is used in white paints, particularly for exterior use. In nature, barium chloride is poisonous.
Properties
It is a white solid which is water-soluble, hygroscopic and gives a slight yellow-green colour to a flame. It is white, highly toxic, and imparts a yellow-green color to a flame, like most other water-soluble barium salts.
- Chemical formula: BaCl2
- Molar mass: 208.23 g/mol (anhydrous); 244.26 g/mol (dihydrate)
- Appearance: White solid
- Odor: Odourless
- Density: 3.856 g/cm3 (anhydrous); 3.0979 g/cm3 (dihydrate)
- Melting point: 962 °C (1,764 °F; 1,235 K) (960 °C, dihydrate)
- Boiling point: 1,560 °C (2,840 °F; 1,830 K)
- Solubility in water: 31.2 g/100 mL (0 °C); 59.4 g/100 mL (100 °C)
- Solubility: soluble in methanol, insoluble in ethanol, ethyl acetate

Structure and properties
BaCl2 crystallizes in two different forms (polymorphs). The cubic fluorite (CaF2) structure dominates one form, while the orthorhombic cotunnite (PbCl2) structure dominates the other. Both polymorphs accommodate the large Ba2+ ion’s preference for coordination numbers greater than six. In the fluorite structure, the coordination of Ba2+ is 8 and in the cotunnite structure, it is 9. When pressures of 7–10 GPa are applied to cotunnite-structure BaCl2, it transforms into a third structure, a monoclinic post-cotunnite phase. Ba2+ coordination number increases from 9 to 10.
Solution
Water dissolves barium chloride quite well (as is the case with most ionic salts). In its dissolved state, it is known to dissociate into barium cations and chloride anions. At 20 degrees Celsius, the solubility of barium chloride in water is approximately 358 grams per litre. However, the solubility of this compound in water varies with temperature. At 100 degrees Celsius, the solubility of barium chloride in water is 594 grams per litre. This substance is also soluble in methanol.
In aqueous solution BaCl2 behaves as a simple salt; in water it is a 1:2 electrolyte and the solution exhibits a neutral pH. Its solutions react with sulfate ion to produce a thick white precipitate of barium sulfate.
Ba2+ + SO42- → BaSO4
Oxalate effects a similar reaction:
Ba2+ + C2O42- → BaC2O4
When it is mixed with sodium hydroxide, it gives the dihydroxide, which is moderately soluble in water.
Preparation
On an industrial scale, it is prepared via a two step process from barite (barium sulfate):
BaSO4 + 4 C → BaS + 4 CO
This first step requires high temperatures.
BaS + 2 HCl → BaCl2 + H2S
In place of HCl, chlorine can be used.
In theory, barium chloride can be made from barium hydroxide or barium carbonate. Hydrated barium chloride is formed when these basic salts react with hydrochloric acid.
Uses
Despite its low cost, barium chloride has few applications in the laboratory and industry. In industry, barium chloride is primarily used in the purification of brine solution in caustic chlorine plants, as well as the production of heat treatment salts and steel case hardening.
- It is used as a raw material to produce barium salt.
- It is used in chlorine-alkali industries.
- Used in the manufacturing of rubber.
- It is widely used in oil refining.
- It is used in the papermaking industry.
- It is used in the hardening of steel.
Safety
Barium chloride, along with other water-soluble barium salts, is highly toxic. Sodium sulfate and magnesium sulfate are potential antidotes because they form barium sulfate BaSO4, which is relatively non-toxic because of its insolubility.