Osmotic pressure can be defined as the pressure required stopping completely, the movement of the solvent through the semi-permeable membrane.
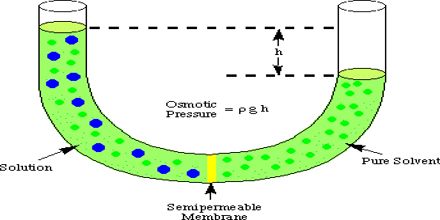
The more is the difference in concentration of the solution, the more is the osmotic pressure.
Osmotic pressure can be defined as the pressure required stopping completely, the movement of the solvent through the semi-permeable membrane.

The more is the difference in concentration of the solution, the more is the osmotic pressure.