Bismuth telluride (Bi2Te3) is a gray powder that is a bismuth and tellurium compound. It is also known as bismuth(III) telluride. It is a semiconductor that is an efficient thermoelectric material for refrigeration or portable power generation when alloyed with antimony or selenium. Because Bi2Te3 is a topological insulator, its physical properties vary with thickness.
Bismuth telluride and its alloys are widely used as thermoelectric refrigeration materials. They are also the best materials for use in thermoelectric generators when the heat source is moderately hot.
Properties
- Chemical formula: Bi2Te3
- Molar mass: 800.76 g/mol
- Appearance: grey powder
- Density: 7.74 g/cm3
- Melting point: 580 °C (1,076 °F; 853 K)
- Solubility in water: insoluble
- Solubility in ethanol: soluble
- Crystal structure: Trigonal, hR15
Properties as a thermoelectric material
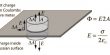
Bismuth telluride is a layered semiconductor with a narrow gap and a trigonal unit cell. The structure of the valence and conduction bands can be described as a many-ellipsoidal model with six constant-energy ellipsoids centered on the reflection planes. Because of Van der Waals bonding between tellurium atoms, Bi2Te3 easily cleaves along the trigonal axis. As a result, bismuth-telluride-based materials used in power generation or cooling must be polycrystalline. Furthermore, the Seebeck coefficient of bulk Bi2Te3 becomes compensated around room temperature, necessitating the use of an alloy of bismuth, antimony, tellurium, and selenium in power-generation devices.
Recently, researchers have attempted to improve the efficiency of Bi2Te3-based materials by creating structures where one or more dimensions are reduced, such as nanowires or thin films. In one such instance, n-type bismuth telluride was shown to have an improved Seebeck coefficient (voltage per unit temperature difference) of −287 μV/K at 54 °C, However, one must realize that Seebeck coefficient and electrical conductivity have a tradeoff: a higher Seebeck coefficient results in decreased carrier concentration and decreased electrical conductivity.
Properties as a topological insulator
Bismuth telluride has been extensively studied as a topological insulator. When its conducting surface states are exposed and isolated, its physical properties change at extremely thin thicknesses. Epitaxy or mechanical exfoliation is used to obtain these thin samples.
Thin Bi2Te3 samples are commonly obtained through epitaxial growth methods such as molecular beam epitaxy and metal-organic chemical vapor deposition. Because the stoichiometry of samples obtained using such techniques varies greatly between experiments, Raman spectroscopy is frequently used to determine relative purity. Thin Bi2Te3 samples, on the other hand, are resistant to Raman spectroscopy due to their low melting point and poor heat dispersion.
Occurrence
Tellurobismuthite is a moderately rare mineral form of Bi2Te3. Many natural bismuth tellurides of various stoichiometry exist, as do compounds of the Bi-Te-S-(Se) system, such as Bi2Te2S. (tetradymite).
Bismuth telluride is easily made by sealing mixed bismuth and tellurium metal powders in a quartz tube under vacuum (critical, as an unsealed or leaking sample may explode in a furnace) and heating it to 800 °C in a muffle furnace.