Bonding in Metallic Crystals and their Characteristic
Depending on the forces that hold the atoms, molecules or ions together in crystal lattice crystals are classified into four main types.
These are: (i) Ionic crystal, (ii) Molecular crystal, (iii) Network covalent crystal and (iv) Metallic crystal. Here briefly focus on Metallic crystal.
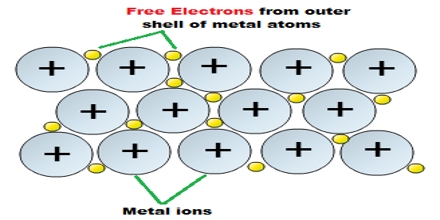
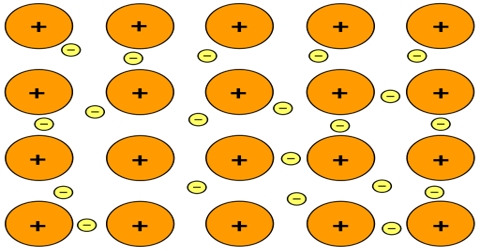
The bonding in metals is quite different from other types of crystals. In a metal the outer electrons of the atoms are delocalized over the entire crystal. The lattice consists of arrays of cations immersed in a sea of valence electrons. Strong electrostatic forces between the canons and the cloud of delocalized electrons are responsible for the greater strength of the metals. The delocalized electrons occupy a series of energy levels. These energy levels are generally very close so as to form a discrete energy band. That are gaps between the energy bands and electrons are forbidden to occupy these gaps. The bands and gaps are characteristic of a solid. The high electrical conductivity of metals may be understood in terms of the Band Model. The ease with which the delocalized electrons manse makes the metals good conductors of heat and electricity. Metals are lustrous (reflectivity), ductile and malleable. All these properties of metal can be explained by, considering delocalization of electrons in metallic crystals.