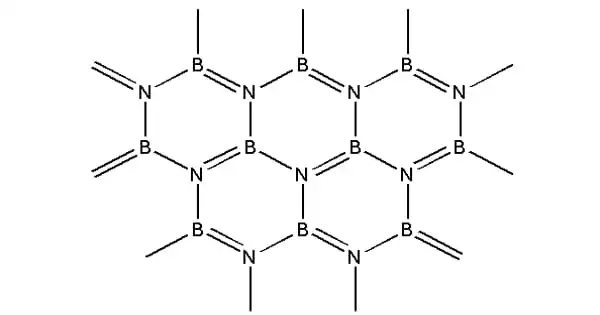
Boron nitride, sometimes known as BN, is a thermally and chemically resistant refractory compound composed of boron and nitrogen. It is a chemical molecule having an equal number of boron and nitrogen atoms that is isoelectronic and isostructural to carbon. It can be found in a variety of crystalline forms that are isoelectronic to a similarly organized carbon lattice.
Boron nitride (BN) is a crystalline substance with refractory qualities that is heat and chemical resistant. It is formed of boron and nitride. Because it exists in numerous polymorphs, BN has grown into a highly valuable molecule, serving a variety of industries and applications.
Production
Boron nitride is created by combining a boron precursor (either boric acid or boron trioxide) with a nitrogen-containing reagent (urea or ammonia) in a nitrogen atmosphere. This reaction produces amorphous boron nitride with trace levels of boron trioxide impurities, which can be refined further by evaporation at temperatures above 1500°C.
Form
Because the hexagonal form corresponding to graphite is the most stable and soft of the BN polymorphs, it is employed as a lubricant and cosmetic component. The cubic (zincblende aka sphalerite structure) variant similar to diamond is known as c-BN; it is softer than diamond but has higher thermal and chemical resilience. The uncommon wurtzite BN modification resembles lonsdaleite but is significantly softer than the cubic form.
Like diamond, the cubic form of BN has a considerably high electrical resistance and thermal conductivity. It does not dissolve in steel components, making it an excellent abrasive substance. In terms of structure and characteristics, the non-crystalline form of boron nitride is equivalent to amorphous carbon. Despite its ultra-thinness, this BN polymorph has strong thermal conductivity, enhanced surface adsorption, and good dielectric characteristics.
When interacting with acids, boron nitride is normally neutral. General acids do not exfoliate well, but utilizing a high protic acid, such as methyl sulfonic acid (MSA), exfoliation was significantly more successful.
Properties
- Resistance to heat and chemicals: The compound has a melting point of 2,973°C and a thermal expansion coefficient that is much higher than that of a diamond. Even when exposed to 1000°C in ambient air, its hexagonal shape resists disintegration. Boron nitride is not soluble in ordinary acids.
- Thermal conductivity: Boron nitride has a thermal conductivity of 1700 to 2000 W/mK, which is equivalent to graphene, a similarly hexagon-latticed material composed of carbon atoms.
- Lubricating property: Boron nitride has the ability to increase the coefficient of friction of lubricating oil while decreasing wear.
- Density: Its density varies depending on its form, although it ranges from 2.1 to 3.5 g/cm3.
Boron nitride is a crystalline substance manufactured in electric furnaces at temperatures ranging from 1,800°C to 2,500°C (3,300°F–4,500°F) from boric anhydride and pure low-ash carbon material. It has a hardness of around 3,800 HV and good cutting capabilities in the form of loose grains. However, boron nitride cannot be used for grinding wheels due to its low oxidation temperature of 430°C (800°F). It is only used in pastes for sintered carbide lapping or as grit for sandblasting.
Boron nitride (BN) ceramics are chemically robust and resistant to molten metals, have good thermal stability in the air, and exhibit anisotropic thermal conductivity, making them suited for broad usage in the production of high-temperature crucibles. BN can exist in multiple phases, and the hexagonal BN (hBN) phase is stable at room temperature. Because of excellent thermal and chemical stability, boron nitride ceramics are used in high-temperature equipment. Boron nitride has potential use in nanotechnology.