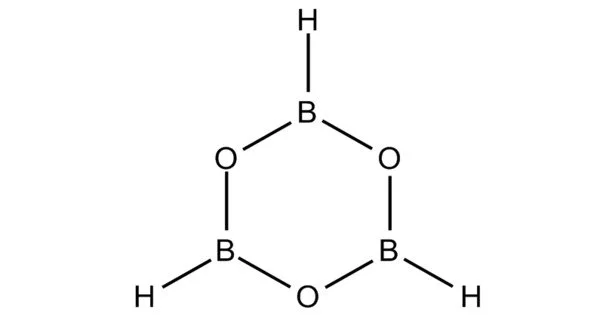
Boroxine (B3H3O3) is a heterocyclic compound with six members made up of alternating oxygen and singly-hydrogenated boron atoms. It is a six-membered inorganic heterocycle with three boron atoms and three oxygen atoms. Boroxine derivatives (boronic anhydrides) such as trimethylboroxine and triphenylboroxine are also members of the boroxine class of compounds. At room temperature, these compounds are solids that are usually in equilibrium with their respective boronic acids. Aside from theoretical studies, boroxine is primarily used in the manufacture of optics.
Properties
- Chemical formula: B3H3O3
- Molar mass: 83.455 g mol−1
Structure and bonding
Because three-coordinate boron compounds typically exhibit trigonal planar geometry, the boroxine ring is also locked in a planar geometry. These compounds have the same isoelectronic properties as benzene. They may have aromatic properties due to the vacant p-orbital on the boron atoms. Boron single bonds on boroxine compounds are mostly of the s variety. B-O bond lengths in ethyl-substituted boroxine are 1.384 Å and B-C bond lengths are 1.565 Å. The bond lengths of phenyl-substituted boroxine are similar at 1.386 Å and 1.546 Å, indicating that the substituent has little effect on the boroxine ring size.
The crystal structure of a boroxine ring is determined by substitutions onto it. The crystal structure of alkyl-substituted boroxines is the most basic. These molecules stack on top of one another, aligning an oxygen atom from one with a boron atom from another, with each boron atom sandwiched between two other oxygen atoms. The individual boroxine rings combine to form a tube. The intermolecular B-O distance of ethyl-substituted boroxine is 3.462 Å, which is significantly greater than the 1.384 Å B-O bond distance.
Phenyl-substituted boroxine has a more complex crystal structure. The interaction of vacant p-orbitals in boron atoms with π-electrons in aromatic, phenyl-substituents results in a different crystal structure. One molecule’s boroxine ring is sandwiched between two phenyl rings from other molecules. The phenyl-substituents can donate π-electron density to the vacant boron p-orbitals thanks to this arrangement.