Buffer Solutions
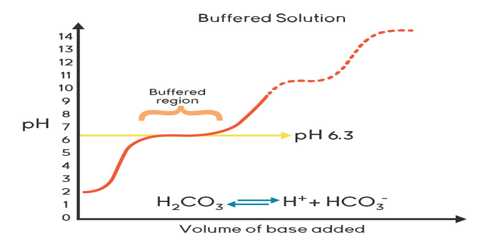
In many chemical and biological processes, it is necessary to maintain the pH of the medium fairly at the constant value. Solutions used for this purpose are known as buffer solutions. A buffer solution is one which resists changes in pH when small quantities of an acid or an alkali are added to it.
“Buffer solution is defined as a solution that resists change in pH upon addition of small quantities of strong acids or strong bases to it”.
A buffer solution has to include things which will eliminate any hydrogen ions or hydroxide ions that you might add to it – otherwise, the pH will transform. Acidic and alkaline buffer solutions achieve this in dissimilar ways. They are buffered by the presence of weak acid and a conjugate base. Buffered solutions resist varying in pH when acids or bases are added to it by reacting with any added hydrogen or hydroxide ions so that they do not accumulate. Therefore, buffered solutions are used to keep pH at a constant value
If 1.0 mL of 1.0 mol L-1 HCl solution is added to 1 L of water the pH changes from 7.0 to 3.0, which mean that the pH changes by 4 units. On the other hand, it can be shown that when the same amount of acid is added to 1 L of an appropriate buffer solution the pH changes only slightly, by 0.1 units or so.