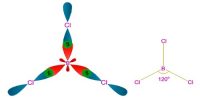
Due to the electrostatic attractive between opposite poles and repulsion between similar poles the molecules orient themselves in such a way that the negative pole of a molecule remain near the positive pole of another molecule.
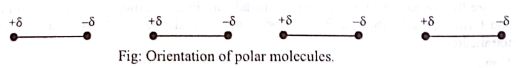
So the polar molecules attract one another and the attractive force among them is known as dipole-dipole interaction.