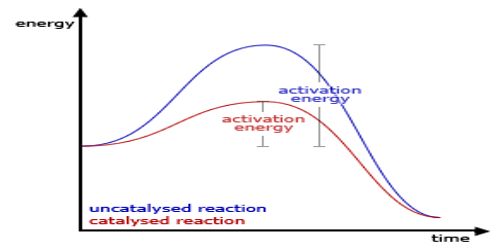
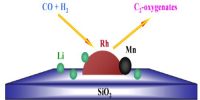
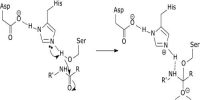
A catalyst is specific in its action. All material bodies cannot act as catalysts in all reactions. A suitable catalyst for a particular reaction is to be found out by trial and error because with our present knowledge a prediction as to the specificity of a catalyst cannot be made. Examples are the different catalysts for different reactions described so far. A catalyst which can be highly reactive in a certain reaction may not be of any use in another reaction.
Extensive research in the field of catalysis has yielded results from which the following claims are now made.
Catalysts can be designed to-
(i) help initiate reaction;
(ii) stabilize the intermediates of a reaction;
(iii) hold the reactant molecules close to each other;
(iv) hold the reactant molecules in the right orientation on the catalyst;
(v) block side reactions,
(vi) make bond easer to break;
(vii) donate and accept electrons and
(viii) act as efficient means tor energy transfer.