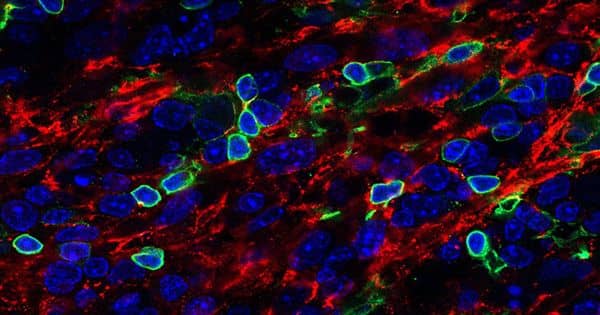
A UCLA-led research team has discovered a chemical cocktail that allows for the production of a large number of muscle stem cells, which can self-renew and give rise to all types of skeletal muscle cells. The breakthrough could pave the way for the development of stem cell-based therapies for muscle loss or damage caused by injury, aging, or disease. The findings were published in the journal Nature Biomedical Engineering.
Muscle stem cells are in charge of muscle growth, repair, and regeneration after injury all throughout a person’s life. Muscle stem cells are dormant in fully grown adults, remaining dormant until they are activated to respond to injury by self-replicating and producing all of the cell types required to repair damaged tissue.
A research team has identified a chemical cocktail that enables the production of large numbers of muscle stem cells, which can self-renew and give rise to all types of skeletal muscle cells.
However, as people age, their regenerative capacity declines; it can also be harmed by traumatic injuries and genetic diseases such as Duchenne muscular dystrophy. “Muscle stem cell-based therapies show a lot of promise for improving muscle regeneration,” said Song Li, the study’s senior author and a member of UCLA’s Eli and Edythe Broad Center of Regenerative Medicine and Stem Cell Research.
Li and his colleagues discovered a chemical cocktail containing the root extract forskolin and the small molecule RepSox that can efficiently generate large numbers of muscle stem cells in 10 days. The researchers demonstrated two potential avenues for using the cocktail as a therapy in mouse studies.
The first method employs dermal myogenic cells, which are skin cells with the potential to become muscle cells. The researchers discovered that treating dermal myogenic cells with the chemical cocktail stimulated the production of a large number of muscle stem cells, which could then be transplanted into injured tissue.
The approach was tested in three groups of mice with muscle injuries: adult (8-week-old) mice, elderly (18-month-old) mice, and adult mice with a genetic mutation similar to the one that causes Duchenne in humans. The muscle stem cells had integrated into the damaged muscle four weeks after the cells were transplanted and significantly improved muscle function in all three groups of mice.
Li’s team used nanoparticles to deliver the chemical cocktail into damaged muscle tissue in the second method. The nanoparticles, which are about one-hundredth the size of a grain of sand, are made of the same material as dissolvable surgical stitches and are designed to slowly release chemicals as they degrade.
In all three types of mice, the second approach produced a robust repair response. When the nanoparticles were injected into the injured muscle, they migrated throughout the area and released chemicals, causing the dormant muscle stem cells to divide. While both techniques were successful, the main advantage of the second was that it eliminated the need for cell growth in the lab; all muscle stem cell activation and regeneration occurs within the body.
The researchers were particularly surprised to discover that the second method was effective even in elderly mice, despite the fact that the environment that surrounds and supports muscle stem cells becomes less effective as animals age. “Our chemical cocktail enabled muscle stem cells in elderly mice to overcome their adverse environment and launch a robust repair response,” said Li, chair of bioengineering at UCLA Samueli School of Engineering and professor of medicine at UCLA David Geffen School of Medicine.
In future studies, the research team hopes to replicate the findings in human cells and to track the effects of the therapy in animals for a longer period of time. The experiments should aid in determining whether either approach could be used as a one-time treatment for patients with severe injuries.
Li pointed out that neither approach would correct the genetic flaw that causes Duchenne and other genetic muscular dystrophies. However, the team believes that muscle stem cells derived from a healthy donor’s skin cells could be transplanted into a muscular dystrophy patient’s muscle, such as the lungs, extending their lifespan and improving their quality of life.