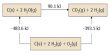
The time required to disintegrate one half of any radioactive substance is called half life period (t½). The half life period (t½) of a radioactive substance is independent of initial concentration. It depends only on the disintegration constant (λ) of the radioactive element. t½ is used to indicate the relative stability of radioactive substance. If t½ is the shorter, faster is the rate of decay and hence the substance is more unstable and viceversa.
t½ = (0.693/λ)
Average life, τ (Tau) = 1/λ = (t½ /0.693) = 1.44 t½